News Brief: 2016 brings four new elements
All had been suspected of being real, but until now lacked formal confirmation
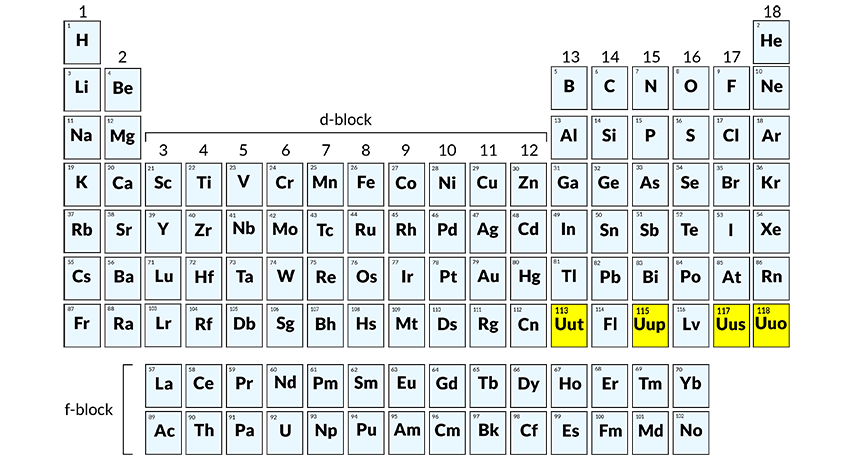
The official discovery of elements 113, 115, 117 and 118 (shown in yellow) means that all chemicals in the periodic table’s first seven rows have been found on Earth or produced in the lab.
E. OTWELL
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
By Andrew Grant
All of the known elements in the universe are represented by boxes on what is known as the Periodic Table. The seventh row of that table is now officially full.
On December 30, the International Union of Pure and Applied Chemistry announced that it had received enough evidence to confirm the existence of four new elements. They are those with the atomic numbers 113, 115, 117 and 118. The group gave credit for the three bigger elements to a Russian-U.S. team. IUPAC awarded credit for finding element 113 to scientists at RIKEN in Wako, Japan.
An element’s atomic number refers to the number of protons in its central core, or nucleus. Those protons are subatomic particles carrying a positive charge. They are usually balanced out by an equal number of electrons orbiting the nucleus. Each of those electrons carries a negative charge.
Scientists had created each of the four new elements. They did this by slamming the nuclei of smaller elements into one another. This created super-heavy elements. All were radioactive and short-lived. As they decayed, they turned into smaller elements.
IUPAC gave bragging rights for 115 and 117 to researchers at the Joint Institute for Nuclear Research in Dubna, Russia, Lawrence Livermore National Laboratory in California and Oak Ridge National Laboratory in Tennessee. The Russian and Livermore researchers also got credit for element 118. Scientists at those two places also had claimed to have made element 113 in 2004 and 2007. But IUPAC instead gave the Japanese group credit for element 113. It will be the first discovered and named by scientists in Asia.
Receiving credit for the new biggies softened the blow of now losing out in credit for 113, says Dawn Shaughnessy. She leads the experimental nuclear and radiochemistry group at the Livermore lab. “I’m personally very happy with IUPAC’s decision,” she says.
Published reports on the four new elements will appear in early 2016, IUPAC says. Official recognition that they exist means that their discoverers will have the right to suggest names and symbols for the elements. For now, each has only a place-holder name. For 113, it’s ununtrium. That’s abbreviated as Uut. The rest are temporarily named ununpentium (Uup), ununseptium (Uus) and ununoctium (Uuo).
Power Words
(for more about Power Words, click here)
atomic number The number of protons in an atomic nucleus, which determines the type of atom and how it behaves.
decay See radioactive decay.
electron A negatively charged particle, usually found orbiting the outer regions of an atom; also, the carrier of electricity within solids.
element (in chemistry) Each of more than 118 substances for which the smallest unit of each is a single atom. Examples include hydrogen, oxygen, carbon, lithium and uranium.
nucleus Plural is nuclei. (in physics) The central core of an atom, which contains most of its mass.
periodic table of the elements A chart (and many variants) that chemists have developed to sort elements into groups with similar characteristics. Most of the different versions of this table that have been developed over the years tend to place the elements in ascending order of their mass.
proton A subatomic particle that is one of the basic building blocks of the atoms that make up matter. Protons belong to the family of particles known as hadrons.
radioactive An adjective that describes unstable elements, such as certain forms (isotopes) of uranium and plutonium. Such elements are said to be unstable because their nucleus sheds energy that is carried away by photons and/or and often one or more subatomic particles. This emission of energy is by a process known as radioactive decay.
radioactive decay A process by which an element is converted into a lighter element through the shedding of subatomic particles (and energy).
radiochemistry A field of research that studies the structure and chemical attributes of radioactive elements and the compounds they form. Scientists who work in this field are known as radiochemists.






