Scientists probe new ways to control malaria
From jungles to mountains, researchers hunt new approaches to tackling this mosquito-borne killer
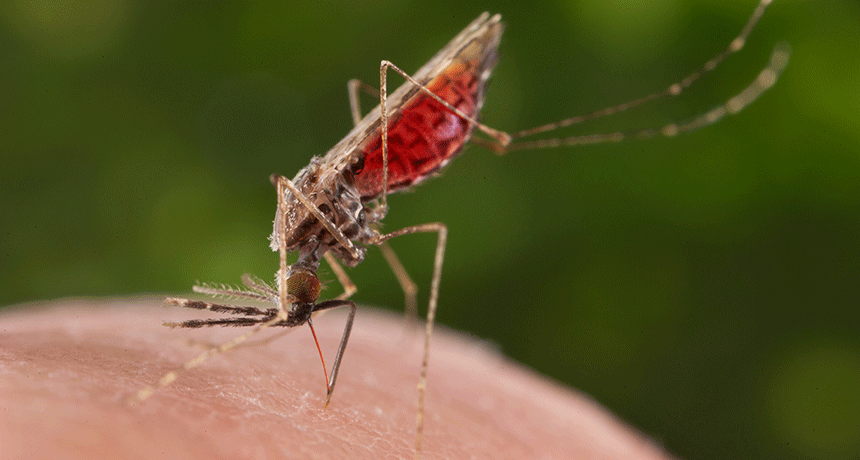
A hungry Anopheles merus mosquito sucks blood from a human hand. This is one of the mosquito species that can spread malaria.
James Gathany/CDC
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
CHICAGO, Ill. — Science detectives are following new clues in their quest to stop a killer — malaria. The disease claimed the lives of roughly 429,000 people in 2015 alone, according to the World Health Organization. And kids face the biggest risk. More than seven in every 10 of malaria’s victims are children.
The disease is caused by a parasite that spends part of its life cycle in mosquitoes and part in blood-making animals. Once it gets into people, it attacks liver and blood cells. Mild cases cause fever, headaches and chills. Severe cases leave the blood with too few healthy cells to carry oxygen. Sometimes, the disease also affects the lungs and brain.
Some medicines can cure malaria. But those drugs don’t always work. So scientists are exploring new ways to thwart this killer. Last summer, they described two of them at a meeting here in Chicago, Ill.
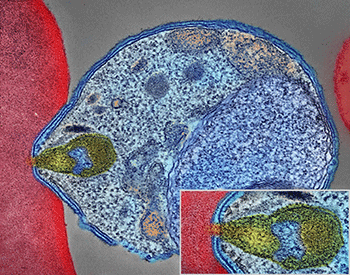
“Malaria doesn’t just affect humans,” explains Holly Lutz. She works in Chicago at the Field Museum and the University of Chicago. Bats, other mammals, birds and lizards also can develop the disease. “In fact, nearly all terrestrial vertebrates can be infected with their own species of malaria,” she says.
Lutz calls herself “both an evolutionary biologist and a microbial ecologist. But first and foremost, I am an explorer,” she says. By exploring animals and their parasites, Lutz hopes to learn more about how to thwart malaria in people.
A microscopic parasite called Plasmodium (Plaz-MO-dee-um) is behind the illness. Mosquitoes pick up the microbe when they feed on infected blood. It grows in their gut. Then it gets into the insects’ saliva. When a mosquito takes its next bite, it can now share some of these parasites with a new host. In many cases, that host may be a human.
There is some good news. Malaria killed only about half as many people in 2015 as it did 15 years earlier. Bed nets helped. People drape these mesh curtains, treated with mosquito-killing poison, over their beds at night. Medical care is better in some areas, too.
Story continues below video
But here’s the bad news. Like other organisms, the parasites that cause malaria evolve. As a result, more of them can now resist the microbe-killing action of various drugs. Mosquitoes also evolve. Some can now resist common insecticides. That means they can survive to infect more people — and reproduce. And climate change is altering weather patterns around the globe. In the future, malaria-carrying mosquitoes will likely survive in more places.

Here in Chicago, two researchers are taking very different approaches to dealing with the problem. Lutz looks at malaria in wildlife. University of Chicago ecologist Mercedes Pascual asks how things like climate change or human sprawl might affect malaria’s spread.
For the birds?
Lutz’s research has taken her to remote parts of Africa, Central America and South America. She wants to know how wildlife deal with malaria. She hopes that what she learns will offer “insights into our own human struggle with this devastating parasite.”
For example, in the East African nation of Malawi, her team collected blood from 152 bird species. Those blood samples showed links between malaria infection rates and the behaviors of different types of birds. For example, they showed two years ago, malaria parasites were more likely to infect bird species that built closed-cup nests.
In her lab, Lutz studies the malaria parasites. These single-celled microbes live inside blood cells. To study the parasites, she has to get them out. Her team recently developed a way to do that. They use a laser to cut a single parasite cell away from the rest of a host’s blood cell.
With the microbes now freed, the researchers can study the genes in the parasites’ DNA.
For one project, Lutz looked at DNA from about 250 Plasmodium species. They included every species known at that time to cause malaria. Many had been discovered in African birds and bats. “Using the DNA from these parasites,” she explains, “I was able to build a ‘family tree’ of malaria to see which parasites were more closely related to each other.” Such closely related species had more similarities in their genes — the codes that instruct cells on what they are to do.
The results offered new clues to where the parasites came from. “This [family] tree,” Lutz reports, “now shows us that the very first mammals to be infected by malaria parasites appear to be bats.” And the types that today infect rodents and other non-human mammals seem to have evolved from those bat-hosted parasites.
More questions remain. For example, in some bird species, up to eight in every 10 individuals may become infected with malaria. Still, many of these animals appear healthy, Lutz says. Why don’t those birds get sick? She hopes to find out.
Lutz also wants to know which plasmodium genes show up in the species that infect people and birds. The answer could point to traits that the parasite needs for survival. And those features could become targets for new malaria medicines, Lutz explains.
On the edges
Mercedes Pascual studies how organisms relate to one another and to their surroundings. In particular, she looks at how changes in the environment can affect the spread of malaria and other diseases.
Malaria thrives in warm areas where rainy seasons make it easy for mosquitoes to breed. As the world continues to warm, more regions will host such conditions. Pascual therefore wondered how climate change might affect outbreaks of malaria in people.
To find out, her team analyzed data on malaria infections from highland areas of Ethiopia and Colombia.
In those areas, not everyone carries the disease. Instead, outbreaks happen from time to time. During an outbreak, lots of people get the disease around the same time. Later, illness rates drop back down. That’s different from many warm places closer to sea level. Although many people have the disease in those places, it’s rare to have huge swings in malaria rates. Instead, the parasites hang out and continually infect a large number of people.
In early 2014, Pascual’s team used a computer to probe how malaria infection relates to climate cycles. They used a computer model to study one cycle known as El Niño (Neen-yo). Every few years, there is a warming of tropical surface waters in parts of the eastern and central Pacific. In later years, those waters cool again off the coast of South America, and warmer water shifts westward. This whole cycle affects weather patterns across the globe. And, Pascual’s team showed, it also affects malaria rates in people. Malaria rates rose as El Niños emerged. Then infection rates fell as that an El Niño ended.
After each El Niño cycle, however, rates of malaria infections generally did not fall all the way back to the earlier background low. The reason, researchers now suspect, is climate change. Higher average temperatures mean parasites have a better chance to survive in high-altitude areas (which are normally cooler regions). If more malaria parasites survive, they can infect more people.
Another one of Pascual’s studies looked at malaria in areas into which people were starting to migrate. People often change the landscape as they settle some new area. They cut down forests. They build roads. Often they divert stream water to irrigate their farms. All of these things can create more spots for water to collect. And that means more places for mosquitoes to breed.
What’s more, as people open new frontiers, they often have few doctors or clinics nearby to treat malaria.
Pascual’s group got data on malaria rates and the new settlements of people in two areas. One was in Brazil. The other was in India. And as people made changes to the land, rates of malaria also varied. The team used math to model what happened as people altered the ways land was used. As rates of land-use changes went up, so did rates of disease.
This work suggests how people might better attack malaria in developing countries. Spraying insecticides and taking other steps to control mosquitoes could prevent many malaria cases as people first move in. As an area becomes more developed, other improvements — such as better medical treatment and housing — could help fight a spread of the disease. The team described its findings in the March 20, 2017, Nature Ecology & Evolution.
A clearer picture

Rickard Ignell is an ecologist at the Swedish University of Agricultural Sciences in Uppsala. He did not work on the projects led by Lutz and Pascual. He thinks Lutz’s work will likely uncover features that are shared by malaria parasites that infect both people and other animals. For example, some genes keep showing up in lots of parasite species. If so, whatever they tell the cells to do might be something the parasites find really, really important. A medicine that targets that feature might kill the parasites.
Pascual’s research is helping to show how malaria spreads, Ignell points out. “With ongoing climate change, we are likely going to see a spread of malaria into regions previously not affected by the disease.” And that can only worsen as people continue to change landscapes, Ignell adds.
S. Noushin Emami is a biologist at Stockholm University in Sweden. She sees some limitations to all of this work. For example, Pascual’s studies don’t deal with all factors that affect the spread of malaria. As for Lutz’s work, she notes that the biology of parasite species can differ from host to host. So findings from one species might not apply to all others.
Still, the work by both teams could give important clues to the mysteries of a major infectious killer, she says. “Malaria is a disease that was and still is very important to a lot of people around the world,” she notes. So each new piece of evidence could give scientists a better picture of how the parasites live and cause disease. Ultimately, that work could help slow — or even stop — this global killer.
Reporting for this piece was made possible by an MBL Logan Science Journalism Fellowship in Chicago arranged by the Marine Biological Laboratory in Woods Hole, Mass., and the University of Chicago.







