Experiment: Where does a bouncing basketball’s energy go?
Can energy loss to heat explain why a bounced ball doesn’t reach its original height?
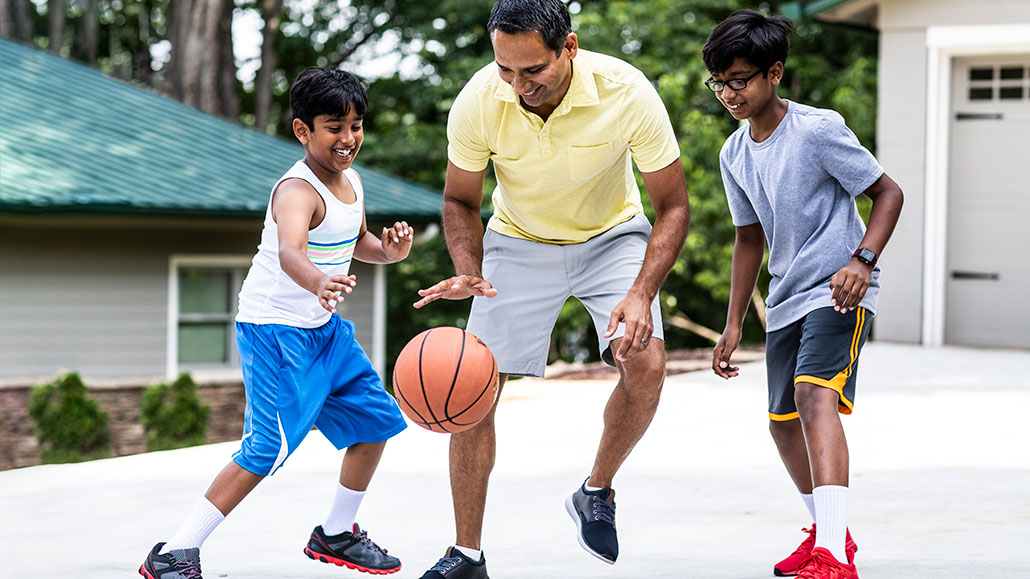
If you drop a basketball and let it bounce on its own, it will quickly lose height. That’s why basketball players must repeatedly push down on, or dribble, a basketball to keep it at a level height.
MoMo Productions/DigitalVision/Getty Images
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
Objective: Determine whether a bouncing basketball’s energy is lost to making heat
Areas of science: Sports science
Difficulty: Medium intermediate
Time required: 2–5 days
Prerequisites: Must be able to dribble a basketball 100 times in a row quickly
Material availability: An infrared thermometer is required to do this science project. See the Materials and Equipment list for details on where to buy one.
Cost: Low ($20–$50)
Safety: No issues
Credits: Teisha Rowland, PhD, Science Buddies
Bounce, bounce, swish! Playing a game of basketball is hard work, and one part of that workout comes from just dribbling the ball. Why does it take effort to dribble the ball? When the basketball hits the ground (and as it flies through the air), the ball actually transfers some of its energy to another form. If a player does not put enough energy back into the ball, they will not be able to dribble it effectively.
When a basketball bounces, it has two different types of energy: kinetic energy and potential energy. Kinetic energy is the energy an object has due to being in motion. Any object that is moving has kinetic energy. A fast-moving basketball has more kinetic energy than a slow-moving basketball. But a basketball that is not moving at all has no kinetic energy.
Potential energy is the energy stored in an object due to its height above the ground. A basketball resting on the floor has no potential energy. When you hold a basketball at waist level, it has some potential energy. If you hold it higher, such as up over your head, it has even more potential energy. If you drop the basketball, the force of gravity pulls it down, and as the ball falls, its potential energy is converted to kinetic energy. As the ball gets closer to the ground, its potential energy decreases. But the ball also speeds up, so its kinetic energy increases.

As we mentioned earlier, when the basketball hits the court’s floor, it “loses” some energy. We know that based on the law of conservation of energy, energy cannot be lost, but it can change form. When the basketball hits the floor, some kinetic energy that the ball has is transferred into another form of energy. A collision where kinetic energy is lost (by changing forms) is called an inelastic collision. (On the other hand, an elastic collision is when the kinetic energy is conserved — it is the same before and after the collision.) When a basketball bounces (without being pushed down), it does not go all the way back up to its original height, as shown in Figure 2 below. This is because the basketball had an inelastic collision with the ground. After a few bounces, it stops bouncing completely. The kinetic energy has left the ball!

Where does the “lost” kinetic energy go? There are many other forms of energy that it can turn into. For example, the sound of the ball hitting the ground comes from some of the kinetic energy being changed into sound. Some of the energy from the impact is also absorbed by the court’s surface, and energy also changes form because the basketball briefly changes shape (it flattens slightly) when it hits the ground.
Another form energy can take is heat, also called thermal energy. In this sports science project, you will determine whether any of the energy of a bouncing basketball takes on the form of heat. Will a basketball warm up after you bounce it? If so, how much heat change do you think will occur? Grab a ball and get ready to find out!
Terms and Concepts
- Energy
- Kinetic energy
- Potential energy
- Gravity
- Law of conservation of energy
- Inelastic collision
Questions
- In a typical game of basketball, when does the ball have more kinetic energy than potential energy and vice versa?
- What are some ways in which energy might be transformed when a basketball is bounced on a court’s floor? What forms can the energy take?
- What are some examples of inelastic collisions you have seen? Do you know how the kinetic energy is lost?
- Can you think of examples that demonstrate the law of conservation of energy?
Materials and Equipment
- A hard surface to quickly dribble a basketball on, such as a basketball court or concrete
- Infrared thermometer with a laser pointer so you can aim it accurately
- Thick, insulating gloves
- Basketball
- Optional: Helper to record your data
- Lab notebook
Experimental Procedure
- Read the manual that comes with the infrared thermometer temperature gun so that you know how it works and how to use it. Practice taking the temperature of objects using the infrared thermometer temperature gun and its laser pointer. Specifically, try to hold the thermometer in one hand and take the temperature of an object you hold out in your other hand at arm’s length. You will need to be able to take the temperature of an object like this quickly.
- In your lab notebook, create a data table like Table 1 below. You will record your results in this data table.
- Make enough rows to collect data for 10 trials, where in each trial you take three temperature measurements of the basketball before and after bouncing it.
| Temperature Before Bouncing (°C) | Temperature After Bouncing (°C) | Average Temperature Before Bouncing (°C) | Average Temperature After Bouncing (°C) | |
| Trial 1 | ||||
| Trial 2 | ||||
| etc. | ||||
| Trial 10 | ||||
- Take your lab notebook, basketball, gloves and infrared thermometer to the location you chose with a hard surface on which you can quickly bounce the basketball. You will be bouncing the ball 100 times in a row, so make sure you have enough space to do this safely!
- To make sure the basketball and gloves are the same temperature as their environment, leave them in the testing location for at least 30 minutes before you start testing. Make sure neither is in direct sunlight, because this can heat up the ball and gloves. Also, test outdoors only when the temperature is relatively stable (during the middle of the day, but not in the early morning or evening).
- If you do this experiment indoors, be sure to have permission to bounce the ball inside.
- Put on the thick, insulating gloves.
- Note: Because some of the heat from your hands may transfer to the basketball while you bounce it, you will wear thick, insulating gloves to prevent this.
- Holding the infrared thermometer in one hand and the basketball in your other hand at arm’s length, quickly take the temperature of the basketball, as shown in Figure 3 below. Take the temperature on three different locations on its surface.
- Do not take too long to make these measurements, because holding the ball may warm it up.
- You do not need to pick specific locations — just try to pick them randomly from different spots spread apart on the basketball’s surface (not all in one area), but avoid taking the temperature where you just held the ball.
- If you have a helper, they can help remember the numbers for you (or write them down).
- Set the ball down and quickly record the temperatures you measured in the data table in your lab notebook as the “Temperature Before Bouncing” for trial 1.
- Now bounce the basketball quickly on the hard surface 100 times in a row.
- Note: It is important to dribble the basketball quickly so that you can see the results of energy being transferred from many consecutive bounces (since it can be hard to detect energy lost from just a few bounces).
- Immediately after you have bounced the basketball 100 times, repeat step 5. Record the temperatures you measured in the data table in your lab notebook as the “Temperature After Bouncing” for trial 1.
- Set the basketball down and do not go onto the next step until the temperature of the basketball is similar to what you measured in step 5. This may take five to 10 minutes.
- Repeat steps 5 to 9 at least nine more times so that you have done at least 10 trials total.
- For each trial, calculate the average temperature before bouncing and the average temperature after bouncing. Write your results in your data table.
- For example, if the three temperatures you recorded for a trial before bouncing the basketball were 23.3° Celsius, 22.8 °C and 23.5 °C, the average temperature you would record would be 23.2°C (because 23.3 °C + 22.8 °C + 23.5 °C = 69.6 °C, and this number divided by three is 23.2 °C).
- Make a bar graph of your results.
- Put the different trial numbers on the x-axis and the temperature on the y-axis.
- For each trial, make two bars, one for the average temperature before bouncing and one for the average temperature after bouncing.
- You can adjust the scale on the y-axis so that the temperature range shown covers only the range of temperatures you recorded.
- Analyze your results and try to draw conclusions.
- Looking at your graphs, do you generally see an increase in temperature after the basketball was bounced 100 times compared to its temperature before bouncing?
- Based on your results, when a basketball is bounced, do you think it “loses” any kinetic energy in the form of heat?
- If your results are different from what you expected, can you explain them? Tip: Think about other ways in which the basketball’s kinetic energy might have been lost by being transformed into another kind of energy.
Variations
- As mentioned in the Background of this science project, when a basketball is dropped on a court, some of the ball’s energy is absorbed by the surface of the court. Different surfaces can absorb different amounts of energy from the bounce. How does this affect whether the basketball transforms kinetic energy into heat? To find out, you could repeat this experiment, but do several trials on different surfaces, such as a basketball court, concrete, a wood floor, linoleum, etc. Do some surfaces cause the ball to lose more energy in the form of heat than others?
- Does a basketball lose more kinetic energy to heat the more times it is bounced? For example, if you bounced a basketball 200 times instead of 100 times, would it convert twice as much kinetic energy to heat? Figure out how you would test this and then give it a try.
- How does bouncing a basketball at different temperatures affect whether it loses kinetic energy to heat? You could try storing a basketball in a refrigerator or freezer, or outside on a cold day, and then bouncing it. To compare your results to a warmer basketball, you could bounce one that was stored at room temperature or outside in the sun on a hot day. Design your experiment and try it out!
- You know that a basketball loses kinetic energy when it bounces, but if you let a basketball drop (without dribbling it) just how many bounces can it make before it loses all of its energy and stops bouncing on its own? And how does this change if you alter some factors, such as the surface the basketball bounces on or the drop height? Design an experiment to investigate how many bounces a basketball can make when you drop it and how various factors affect this. You will also want to measure how high each consecutive bounce is. You could then make a graph of bounce height versus bounce number. Give it a try!
- How much heat does the basketball gain from your hands? This process is called heat transfer. To explore heat transfer from your hands to the basketball while you bounce it, repeat this experiment but compare the change in temperature between wearing gloves and not wearing gloves. Does the basketball gain much heat from your hands?
- How do different balls lose kinetic energy in the form of heat when they are bounced? You can try this science project again, but use different types of balls. (You will want ones that are large enough to easily quickly measure their temperature using the infrared gun.) You could test a variety of different types of balls, such as soccer balls, tennis balls, kickballs and more.
- When the basketball hits the ground, energy is transferred to the floor in the form of sound. Design an experiment to investigate one (or more) of the other ways that energy is transferred, such as by measuring how loudly the ball hits the ground, or how much the ball is flattened, when the basketball is just dropped once on the floor. You could even measure the temperature of the floor after the ball has been bounced in one spot many times (100 or more).
- Equation 1 below is a straightforward equation you can use to calculate the gravitational potential energy of a ball. You can use this equation to calculate the potential energy of the basketball when you drop it. You could try using a video camera to record and measure the bounce height of the basketball (by making height markings on a wall in the background) and calculate how much potential energy it has each time it is dropped. How much energy is transferred on each bounce?
Equation 1:
PE = mgh
- PE = potential energy (in joules [J])
- m = mass of the basketball (in kilograms [kg])
- g = acceleration due to gravity, which is 9.81 meters per second squared (m/s²)
- h = height (in meters)
This activity is brought to you in partnership with Science Buddies. Find the original activity on the Science Buddies website.






