What to know as Pfizer’s COVID-19 vaccine rolls out for kids under 12
No unusual side effects after vaccination were reported in trials of young children
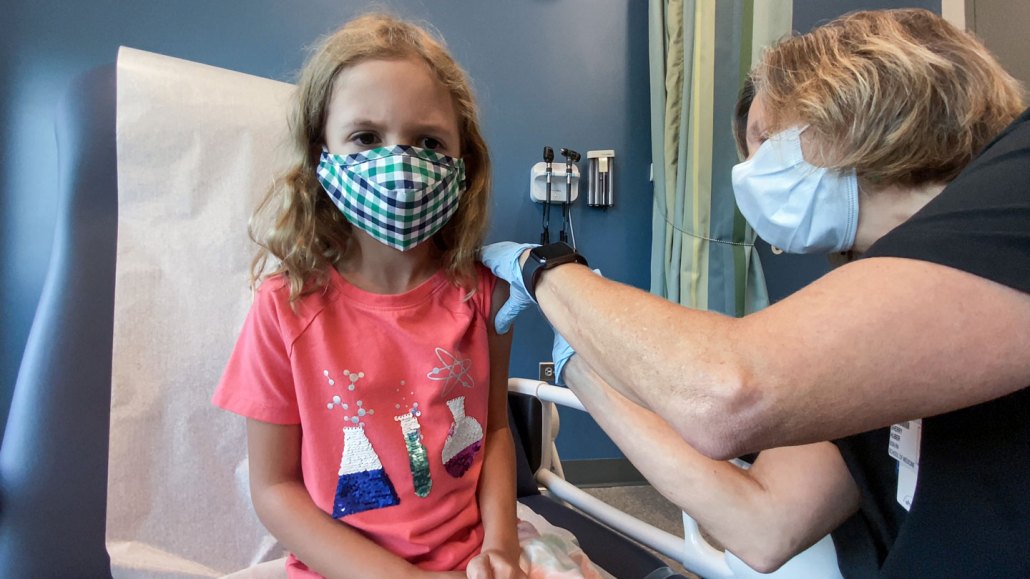
Lydia Melo, 7, gets the first of two vaccinations on September 28, 2021 as part of a clinical trial of Pfizer’s COVID-19 vaccine. An FDA advisory panel endorsed emergency use authorization of this vaccine for kids 5 to 11 years old.
Shawn Rocco/Duke Health
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
Five to 11-year-olds in the United States are at last eligible for a COVID-19 vaccine.
On November 2, federal health officials signed off on the shots for these school-age children. The move followed an October 26 meeting of an advisory panel to the U.S. Food and Drug Administration. That panel gave a thumbs-up to making the vaccine by Pfizer and its German partner BioNTech available to school-age children under 12. A second advisory panel to the U.S. Centers for Disease Control and Prevention met November 2. That panel also said 5- to 11-year-olds could get the vaccine.
In making that decision, experts on the FDA panel had debated whether the vaccine’s benefits outweighed its risks. The CDC panelists discussed the benefits and risks, too. Experts on that panel also developed recommendations for how the vaccine should be used for school-age kids.
For instance, many panelists voiced concern about myocarditis (My-ok-kar-DY-tis). It’s an inflammation of the heart muscle. Although uncommon, myocarditis is a potentially serious side effect of the type of mRNA vaccines made by Pfizer and Moderna. This heart effect showed up primarily in male adolescents and young adults. Other experts emphasized that while children are less likely to develop severe COVID-19 than adults, kids aren’t spared from the worst of the disease.
Amanda Cohn is chief medical officer for vaccine policy at the CDC in Atlanta, Ga. To her, the answer is clear: Vaccine benefits outweigh the risks. “We don’t want children to be dying of COVID, even if it is far fewer children than adults,” she said. “And we don’t want them in the ICU.” ICU is short for a hospital’s intensive care unit.
Jay Portnoy is an FDA panel member who works at Children’s Mercy Hospital in Kansas City, Mo. At the October 26 meeting, he noted his hospital has been full for the last month or so. And many of the kids who were admitted had COVID-19. “I’m looking forward to being able to actually do something to prevent that,” he said.
For nearly two years, kids have faced disrupted school and social lives. Vaccination could bring them a step closer to returning to normalcy. Approving vaccines is “really to allow kids the freedom to be kids,” says Emmanuel Walter Jr. He’s a pediatrician at Duke University School of Medicine in Durham, N.C. He also is the chief medical officer of the Duke Human Vaccine Institute. Once vaccinated, he notes, children can go to school, play sports and “do the normal things kids want to do.”
Vaccinations are key to preventing COVID-19. Here’s what to know about this disease and the Pfizer vaccine in school-age kids under 12.
How was the vaccine evaluated?
Pfizer assessed immunity in kids 5 to 11 who were given the vaccine as part of a clinical trial in about 2,250 children. Two-thirds got the shot. The rest received a placebo.
Children’s immune systems tend to respond well to vaccines. An earlier study had found that this age group had good immunity after two, 10-microgram shots of the vaccine. Each shot is only one-third the dose given older kids and adults. The goal with the smaller dose has been to hit that sweet spot between a protective immune response and any side effects.
How well does the vaccine work?
Trial data suggest the vaccine works very well in the youngest school-age kids. Pfizer assessed this by looking at antibody levels.
The company compared the response in a group of 5- to 11-year-olds one month after their second shots to antibody levels seen in a group of 16- to 25-year-olds. Even though the younger kids had gotten a far smaller dose, there was no difference in antibodies. This suggests the vaccine protects about as well as what’s been seen in the older group.
Pfizer also looked at how many kids who were vaccinated got symptoms of COVID-19 at least a week after the second shot. Three of 1,450 vaccinated children developed the disease. In contrast, 16 of 736 kids getting the placebo did. Pfizer reports this as an efficacy rate for the vaccine of 90 percent.
No child in the trial developed severe COVID-19.
What are the side effects?
In the clinical trial, several common side effects were reported. These include redness, swelling and pain at the site of the injection in the arm. Some young kids also got a headache and became tired for up to a day or so. “That’s to be expected,” Walter said. And if you end up feeling crummy, Walter said, it’s fine to take over-the-counter pain medication.
Should 11-year-olds wait until they’re 12 to get the adult-size dose?
“I would not wait,” said Walter. He’s the Duke pediatrician and a doctor involved in the clinical trial of Pfizer’s vaccine.
Why not wait? Even with the lower dose, 11-year-olds developed lots of antibodies. That’s according to data presented during the October 26 FDA advisory meeting. One month after their second dose, 5- to 11-year-olds had similar antibody levels as older age groups. “I’m confident they’ll have the same protection” as 12-year-olds, Walter says.
How have COVID-19 infections affected 5- to 11-year-olds?
As of October 28, 171 children in this age group in the United States have died of COVID-19. That number comes from CDC’s COVID data tracker. Looking at the top 10 causes of death for children in 2019, COVID-19 would be eighth on that list, says Fiona Havers. She’s a medical officer with the CDC’s COVID-19 Public Health Response group. She spoke during a presentation at the FDA meeting. To date, more than 8,300 U.S. kids ages 5 to 11 have been hospitalized. Almost one in every three of them needed intensive care, she added.
Compared to white children, Havers notes, the hospitalization rate in these younger kids is three times as high for Black, Hispanic and American Indian/Alaska Native children.
James Hildreth is president of Meharry Medical College in Nashville, Tenn. He said the main reason for his yes vote at the FDA advisory panel meeting was to make sure that “the children who really need this vaccine — primarily the Black and brown children in this country — get the vaccine.”
The rate of hospitalization for COVID-19 in 5- to 11-year-olds roughly matches what was seen for flu before the pandemic. But during the COVID-19 pandemic, there has been masking, school closures and other public health measures. Without those precautions, Havers said, the COVID-19 hospitalization rate might have been higher than that for flu in past seasons.
More than 5,000 children who were infected with the coronavirus also have developed multisystem inflammatory syndrome in children, or MIS-C. It’s a severe inflammatory disease that affects the whole body. Of those reported cases, nearly four in every 10 were among children ages 6 to 11, Havers reported.
Children also can experience long COVID. This refers to symptoms (sometimes severe) that last months. These can include fatigue, headaches, difficulty concentrating and pain in the muscles and joints. Such long-lived symptoms can limit activity. They also can hurt a child’s mental health and school attendance. Long COVID can occur after mild or severe disease or following MIS-C. It’s still not clear how many children have experienced long COVID. One estimate from a United Kingdom survey reported that seven to eight in every 100 U.K. children who got COVID-19 still had symptoms more than three months later.
COVID-19 also has kept many kids out of school. This has stalled their learning. For many, it has meant missing out on regular school-provided meals, too. School closures that happened from August to October 2021, as the delta variant was surging, affected more than 1 million U.S. students.
No myocarditis with vaccinations during the trial
No cases of myocarditis occurred in kids ages 5 to 11 in the three months after they were vaccinated. But this rare complication has been seen on occasion among the tens of millions of 12- to 29-year-olds who have now been vaccinated. That’s why weighing the risk of myocarditis against vaccine benefits was a hot topic of discussion at meeting of FDA’s advisory panel.
Of 877 confirmed myocarditis cases in older-vaccine recipients, 829 were hospitalized. None died. As of October 6, 77 percent of adolescents and young adults discharged from the hospital for myocarditis are known to have recovered.
Myocarditis also can occur with COVID-19. Coronavirus patients had 16 times the risk for myocarditis as patients without the disease, according to a September study. It reviewed U.S. hospital data from March 2020 to January 2021.
“We will continue to actively look for adverse events,” said Peter Marks. He directs FDA’s Center for Biologics Evaluation and Research in Silver Spring, Md., and spoke at the FDA advisory-panel meeting.







