A bit of electricity can glue hard metals to soft materials
Playful experimentation in the lab revealed this surprising effect
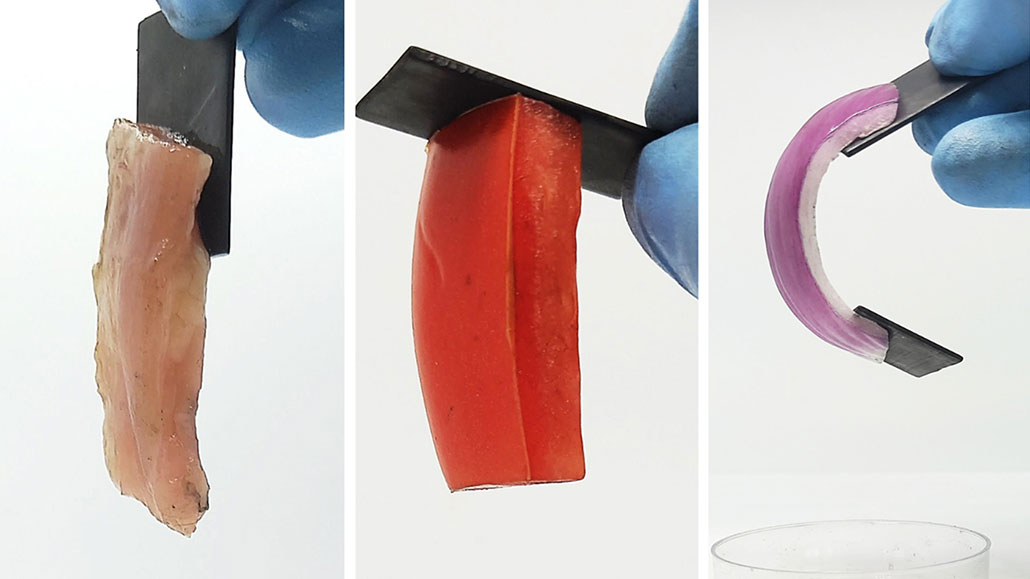
A weak electrical current was able to glue metal pieces to a variety of soft tissues, including a piece of chicken (left), tomato (center) and onion (right).
S Raghavan/University of Maryland
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
When you imagine a scientist, you might picture a serious-looking person peering into vials or frowning at equations. But not all science is serious. In a lab at the University of Maryland in College Park, researchers spend a lot of time playing around with different materials just to see what will happen. That fun, improvised exploration has led to some cool discoveries. The latest: a weak electric current can stick hard metals to soft gels and tissues.
The exact science behind this effect is still a mystery. But the unexpected find could one day be used to create new types of batteries, move robots or attach medical implants to tissues inside people’s bodies.
Chemical engineer Srinivasa Raghavan runs the lab that did this work. His group aims to create soft solids, such as gels with unusual properties, using materials found at home or the grocery store. Their Rapid Seal Wound Gel stops bleeding by knitting blood cells together. You may have some of it in your home. Another one of their gels, made from Jell-O and cornstarch, forms a thin, protective layer that can keep an egg from cracking if dropped.
“The way that we do research is not that different from how it would be done in a middle school or high school,” Raghavan says. “We kind of, you know, mix things and see what happens.”
Do you have a science question? We can help!
Submit your question here, and we might answer it an upcoming issue of Science News Explores
Sticky science
In 2021, the lab created a polymer-based gel that could be stuck to animal tissues. The researchers placed a piece of this gel on a slice of meat and applied a low-voltage electric current to the gel for a few seconds. When the current stopped, the gel and meat had been glued together.
One day, surgeons might use a gel like this instead of stitches or staples to put patients’ tissues back together, Raghavan says.
That electric gel experiment got other lab members thinking about ways to use electricity. Wenhao Xu, a chemist, found some graphite lying around the lab. He decided to see if he could stick it to a piece of gel by running a current through it.
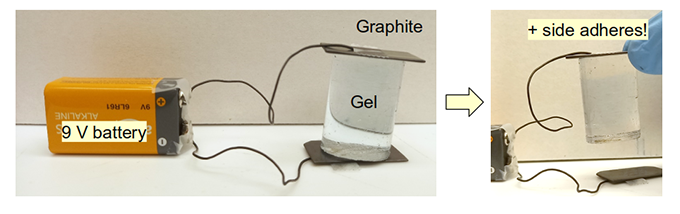
He put two flat pieces of graphite — the stuff found in pencil lead — on opposite sides of a piece of gel. Then he clipped wires onto the graphite pieces and added 10 volts of electricity. For comparison, a 9-volt battery can power a small radio or walkie-talkie.
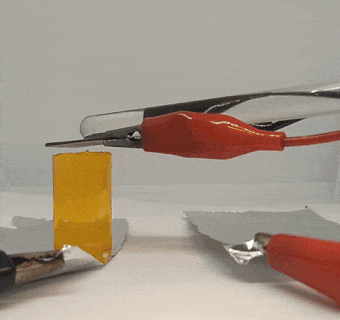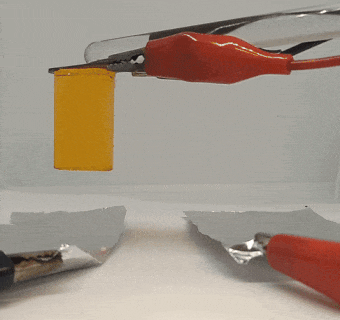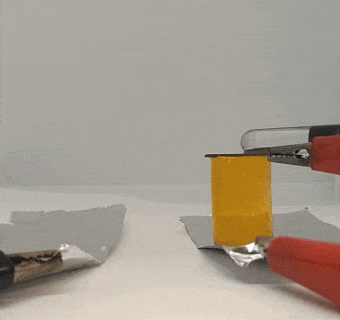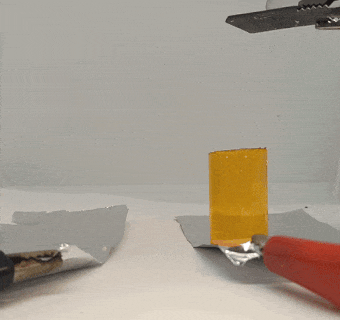
The graphite pieces acted as electrodes, moving electricity through the gel. Electric current ran into the gel through one piece (the positive electrode) and out through the other piece (the negative electrode).
When Xu tried to pull the graphite off of the gel, the positive electrode was thoroughly stuck. The electricity had basically glued it to the gel. Intrigued, Xu placed the other piece of graphite back on the gel and switched the wires so that the current ran through the gel in the reverse direction. When the current stopped, the gel came free from the first piece of graphite it had been stuck to. But now it was stuck to the other piece, which was now the positive electrode.
It was a complete surprise to everyone. “But there are surprises all the time,” Raghavan says. That’s part of the fun of working in the lab.
The plot thickens
Xu next tried replacing the gel with other kinds of materials. He tested out fruits, vegetables and different kinds of meat. He also used a variety of metals for the electrodes. The results were an even bigger surprise.
“Either one side sticks — the plus side sticks or the minus side sticks — or both sides stick,” Raghavan says. “There’s no rhyme or reason.” Beef sticks to the positive, or “plus side” electrode. Pork sticks to the negative, or “minus side” electrode. Jell-O and banana stick to both pieces of metal. And some metals work with some soft materials but not with others.
The team shared its findings on March 13 in ACS Central Science.
“There are lots of things we don’t fully understand,” Raghavan says. The electric field must somehow change the chemistry where the soft and hard materials meet. But the team doesn’t yet know how or why. The idea that the lab has “discovered some secret in nature” is an incredible feeling, Raghavan says.
“I find this work fascinating because of its simplicity,” says Kevin Turner. He is a mechanical engineer at the University of Pennsylvania in Philadelphia who was not involved with the study. Turner is especially impressed the researchers could stick things together underwater, “which is something that is generally hard to do.” Try using a piece of tape underwater — it won’t stick. But the new study shows that electricity can get the job done for certain materials.
Members of the lab continue to play around and experiment with new combinations of materials. “Research should be fun,” Raghavan says. And you don’t have to have special training or use special equipment to do science. “You need to have a sense of curiosity,” he says. “There are lots of questions you can answer even as a middle school student.”






