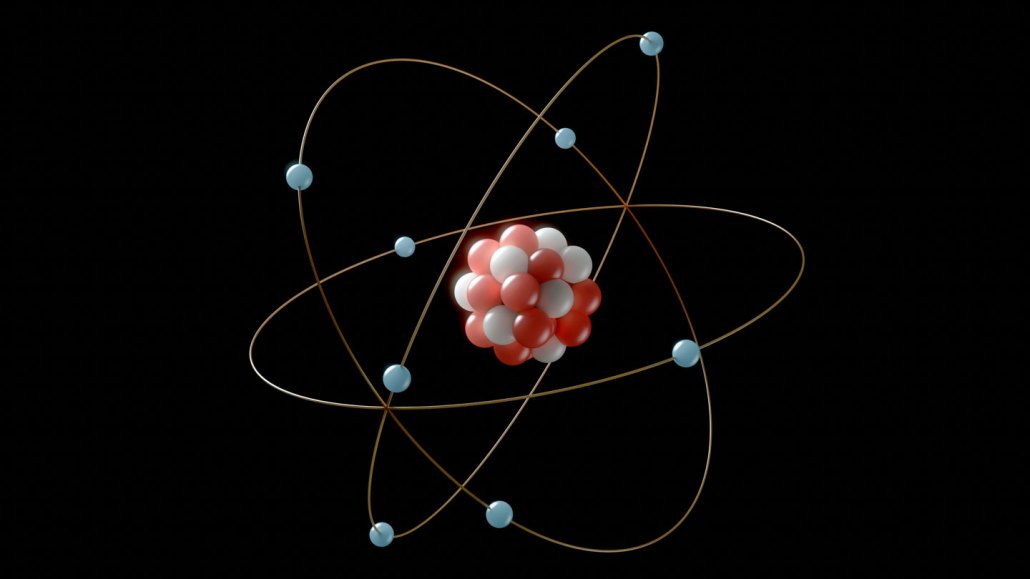
atom: The basic unit of a chemical element. Atoms are made up of a dense nucleus that contains positively charged protons and uncharged neutrons. The nucleus is orbited by a cloud of negatively charged electrons.
cloud: A plume of molecules or particles, such as water droplets, that move under the action of an outside force, such as wind, radiation or water currents.
electric charge: The physical property responsible for electric force; it can be negative or positive.
electron: A negatively charged particle, usually found orbiting the outer regions of an atom; also, the carrier of electricity within solids.
fundamental: Something that is basic or serves as the foundation for another thing or idea.
ion: (adj. ionized) An atom or molecule with an electric charge due to the loss or gain of one or more electrons. An ionized gas, or plasma, is where all of the electrons have been separated from their parent atoms.
link: A connection between two people or things.
mass: A number that shows how much an object resists speeding up and slowing down — basically a measure of how much matter that object is made from.
matter: Something that occupies space and has mass. Anything on Earth with matter will have a property described as "weight."
molecule: An electrically neutral group of atoms that represents the smallest possible amount of a chemical compound. Molecules can be made of single types of atoms or of different types. For example, the oxygen in the air is made of two oxygen atoms (O2), but water is made of two hydrogen atoms and one oxygen atom (H2O).
neutron: A subatomic particle carrying no electric charge that is one of the basic pieces of matter. Neutrons belong to the family of particles known as hadrons.
nucleus: Plural is nuclei. (in physics) The central core of an atom, containing most of its mass.
particle: A minute amount of something.
plasma: (in chemistry and physics) A gaseous state of matter in which electrons separate from the atom. A plasma includes both positively and negatively charged particles.
proton: A subatomic particle that is one of the basic building blocks of the atoms that make up matter. Protons belong to the family of particles known as hadrons.