Pollution power? A new device turns carbon dioxide into fuel
It converts CO2 into a safe, storable salt that can run a fuel cell to make electricity

Cement plants, like the one shown here, are a major source of the carbon dioxide that contributes to climate warming. But some of that pollution could be transformed into a new type of fuel. It’s a salt that can be safely stored for decades or longer.
Witthaya Prasongsin/Moment/Getty Images Plus
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
By Laura Allen
This is another in our series of stories identifying new technologies and actions that can slow climate change, reduce its impacts or help communities cope with a rapidly changing world.
Activities that spew carbon dioxide (CO2) — a common greenhouse gas — have been contributing to the global warming of Earth’s atmosphere. The idea of vacuuming CO2 from the air and stashing it away is not new. It’s just hard to do, especially at a cost people can easily afford. A new system tackles the CO2-pollution problem a bit differently. It chemically converts that climate-warming gas into a fuel.
Researchers at the Massachusetts Institute of Technology (MIT) in Cambridge unveiled their innovation Nov. 15 in Cell Reports Physical Science.
Their new system has two parts. The first makes the fuel by converting airborne CO2 into a molecule called a formate salt. Like CO2, formate has a carbon atom and two oxygens. It also has a hydrogen atom. Formate salts contain some other element, too. The new research uses a type made with either sodium or potassium.
The second part of the system uses that formate salt to power a fuel cell, which makes electricity.
Most fuel cells run on hydrogen. This flammable gas needs pipelines and pressurized tanks to transport it. But fuel cells can also run on formates. Formate salts have as much energy as hydrogen, explains Ju Li. He’s a materials scientist who led the development of the new system. Formate offers some advantages over hydrogen, Li notes. It’s safer and doesn’t need to be stored at high pressure.
The MIT researchers created a fuel cell to test their CO2-derived formate. First, they mixed that salt with water. Then they fed this mix into the fuel cell. Inside, the formate released electrons in a chemical reaction. Those electrons moved from the negative side of the fuel cell to the positive side, creating a circuit. These flowing electrons — electricity — ran for 200 hours in their experiment.
Materials scientist Zhen Zhang, who works with Li at MIT, is optimistic that his team can scale up this new technology within a decade.
The role of CO2
The MIT team used chemistry to transform CO2 gas into the key ingredient to make their fuel. First, they exposed it to a strong alkaline (or basic) solution. They chose sodium hydroxide (NaOH), commonly known as lye. This triggered a chemical reaction that created sodium bicarbonate (NaHCO3), better known as baking soda.
Then they added electricity. It drives a new chemical reaction that cleaves off an oxygen atom from each molecule of the baking soda. This leaves sodium formate (NaCHO2). Their system converted nearly all of the CO2’s carbon — more than 96 percent — into this salt.
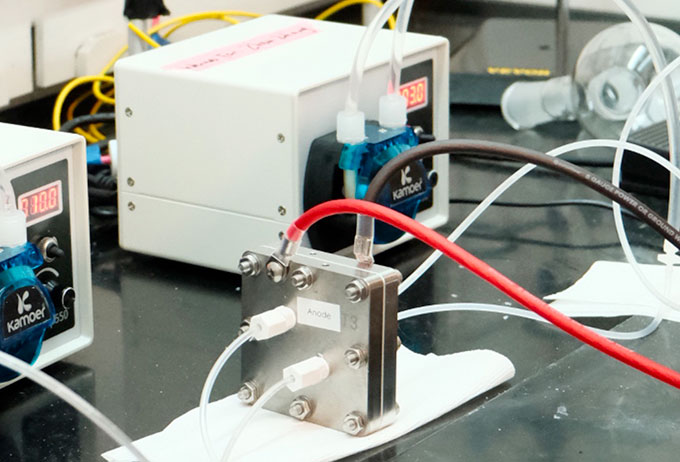
The energy used to remove the oxygen is stored in the formate’s chemical bonds. Formate can store that energy for decades without losing potential power, Li notes. Later, when run through a fuel cell, it can produce electricity. And if the electricity used to make the formate came from solar, wind or hydropower, then the fuel cell’s electricity will be a green form of energy.
To scale up this new technology, “we need to find abundant geological sources of [lye],” says Li. He’s looking at a type of rock called alkali basalt (AL-kuh-lye buh-SALT). When mixed with water, these rocks turn into lye.
Formate’s promise
Farzan Kazemifar is an engineer at San Jose State University in California. His research has focused on storing CO2 underground in salty aquifers. Taking CO2 out of the air has always been a challenge, he says, and therefore costly. So making it into a usable product, such as formate, is attractive, he says. The value of the product could offset the cost of making it.
There’s a lot of research on sucking CO2 from the air. For instance, a team at Lehigh University recently described a different way to filter the gas out of the air and turn it into baking soda. Other groups are storing it in special rocks, turning it into solid carbon and converting it into ethanol, an alcohol fuel. Most of these projects are small-scale. As such, they have done little so far to reduce high levels of CO2 in the air.

Future fuel
This representation imagines a CO2-powered house. A device pictured in front converts CO2 (red-and-white-bubble molecules) into a salt: formate (the blue, red, white and black bubbles). That salt can then power a fuel cell to make electricity.
Our best option, Kazemifar says, would be to “reduce emissions of greenhouse gases in the first place.” One way is to replace fossil fuels with renewables, such as wind or solar power. It’s part of a transition scientists call decarbonization. But, he adds, stopping climate change will take a multi-pronged approach. Capturing carbon, such as with this new tech, will be needed in areas that are hard to decarbonize, he says. Two examples: steel and cement plants.
The MIT group also sees an advantage in partnering its new tech with solar and wind power. Traditional batteries are designed to store power for weeks. To store energy from the summer sun until winter — or longer — takes a different approach. “With formate fuel,” Li says, you’re not even limited to seasonal storage. “It can be generational.”
It may not be as glitzy as gold, but “I can leave my son and daughter 200 tons of … formate,” Li notes — “as an inheritance.”






