A new solar-powered gel purifies water in a flash
The fruit-inspired material could quench thirst where drinking water is scarce
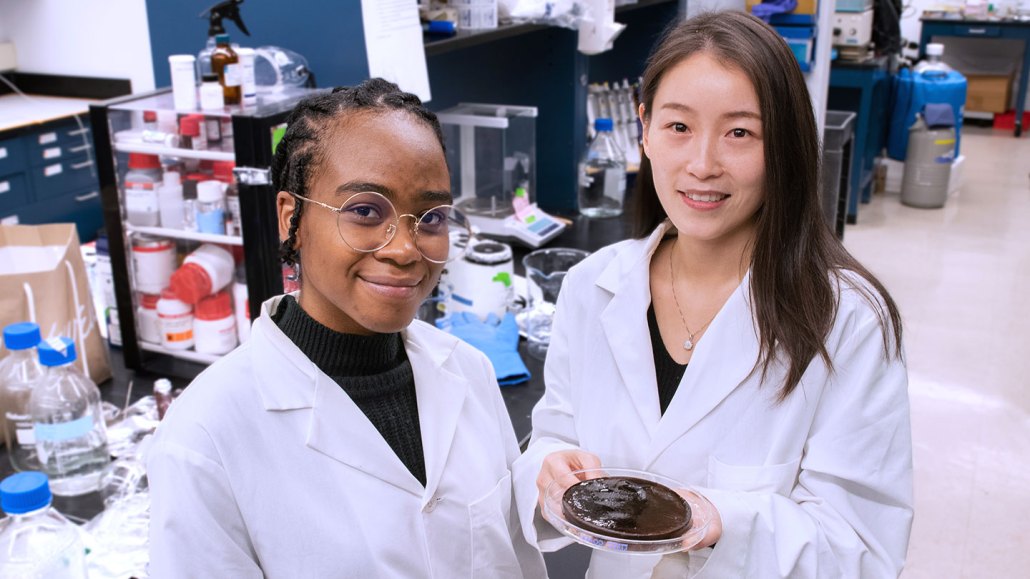
Néhémie Guillomaitre and Xiaohui Xu hold their water-purifying hydrogel. It works by soaking up water and filtering out contaminants.
Bumper DeJesus/Princeton University
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
A new gel can sponge up dirty water. When the water later comes out, it emerges clean and fresh.
The new material is a hydrogel, spongy tangles of threadlike molecules that stick to — and absorb — water. Like a string of beads, it’s made of large molecules called polymers that are strung together from repeating units. A plain old hydrogel sitting in dirty water would get scummy on the outside. Clean water would get dirty again when it flowed out of the gel. But the new hydrogel is self-cleaning.
When dropped in polluted water, the gel absorbs the water. It blocks entry, however, to things that could make someone sick. These include bacteria, oils, heavy metals and salts. A special polymer net throughout the hydrogel repels oil and bacteria from the gel’s surface. So put this gel in water and any oil on the outside immediately jumps off, says Xiaohui Xu. She’s a chemical engineer at Princeton University in New Jersey. Her lab created the new gel.
Allowing the gel to warm up in sunlight self-squeezes out its now-filtered water.
“It may be a great way to make pure water for families,” says Edward Cussler. He’s a chemical engineer at the University of Minnesota in Minneapolis and wasn’t involved in the study. Such a gel might cut risk of disease. Right now, drinking dirty water kills more than 1.5 million people each year.
Researchers described the new material in the February 22 ACS Central Science.
Scum-repelling and super-fast
In the lab, Xu’s team tested the gel’s ability to repel E. coli bacteria. When they pulled the gel out of microbe-tainted water, it held no E. coli hitchhikers. However, Xu points out, other bacteria may be able to stick even if E. coli can’t. That’s why her team is now working on a new version of the gel that does more than block microbes. It will kill them, too.
In an hour, the new gel can clean up about 26 liters (7 gallons) of clean water per square meter of material. Nibedita Nandi thinks this technology could unlock new ways to solve problems of scarce drinking water. Nandi studies biochemistry at the University of Freiburg in Germany. She, too, did not take part in the gel’s creation. It can clean enough water to meet someone’s daily needs for drinking, washing and other household tasks, she says.
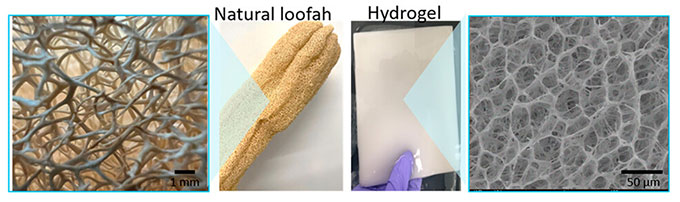
The new material also absorbs and releases water faster than earlier water-purifying gels. The threadlike molecules in most water-cleaning hydrogels trap water inside bubblelike spaces. This can make it hard for water to get out again. But in the new gel, “we created a unique, open-pore structure,” Xu says.
Her team’s inspiration was the loofah, a fruit that becomes spongelike when dried. Tangled molecules inside the new gel look like the loofah’s connected fibers, Xu says. They let water flush out easily.
Powered by sunshine
Water purification often takes a lot of energy. The new hydrogel doesn’t. It runs on sunlight. For Cussler, that’s the coolest part.
Parts of the gel’s threads attract water and other parts repel water, Xu explains. At cooler temperatures, the water-attracting forces are strongest. So, the gel absorbs water.
A black coating helps the gel heat up quickly in sunlight. As the gel warms, its threadlike molecules lose their ability to hold water. Yet as these water-attracting parts weaken, the water-repelling forces stay the same.
The water-attracting forces become weakest at 33º Celsius (91º Fahrenheit). That’s when clean water rushes out.
The gel shrinks when it’s warm and expands when it’s cool. That’s the opposite of how most materials respond to warming. But this shrinking at warm temps explains how the sponge essentially wrings out its water.
That weird property also may make this hydrogel helpful in robotics. Machines built with the gel could behave in ways that devices made with other materials can’t. Imagine a hydrogel robotic hand, says Xu. Temperature changes could cause “the entire structure to conform or to respond in a certain way… maybe to grasp something.”
Cussler has ideas too. He says the gel could be added to some liquids such as milk or orange juice, to dehydrate them. That would make them easier to ship. Or if it could be made sterile, the gel could even be used to remove water from blood for storage or transport.
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.





