Synthetic biology aims to tackle disease and give cells superpowers
These tiny nano-systems can improve or replace our cells’ natural ‘machinery’

Here’s an artist’s concept of what a nano-robot might look like as it attempts to repair damaged proteins in a cell. Tiny machines such as these may one day repair broken cell machinery.
Valentina Kruchinina/iStock/Getty Images Plus
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
Kerstin Göpfrich builds machines. That’s not unusual for an engineer. But rather than hulking metal devices, she makes teeny-tiny ones. They’re designed to work inside our cells.
Göpfrich leads a research group focused on the “engineering of life” at the Max Planck Institute for Medical Research in Heidelberg, Germany. Our cells are already chock-full of nature-made machines. They carry out the daily tasks of living. Many are proteins.
Some proteins allow muscle cells to contract. Others break down our food or help our blood clot. Proteins move materials and enable our immune system to identify and destroy germs.
But sometimes our natural protein-machines don’t work right. Broken bio-machinery underlies many diseases and other health problems. That’s why scientists are looking at how they might fix faulty proteins or even build synthetic replacement parts.
They might repair faulty machinery in the eyes — problems that can result in blindness. Or they might wire up artificial nerves to replace damaged ones.
And the potential for such devices goes beyond making repairs. Scientists are looking at how to give cells new abilities. Some researchers hope that by developing enough novel biomachines, they might even be able to make new forms of life.
The problem with proteins
When some machine breaks — say, part of a car’s engine — we might replace it with a new part to get it up and running again. So if a protein is faulty, why not just make a new one in the lab?
Back when Frankie Rawson was a student, he asked his teachers just that. Now a nanotechnologist at the University of Nottingham in England, Rawson has answered his own question.
“Turns out,” he says, we’re “not very good at artificially re-creating what biology does.” Making proteins in the lab is hard — and for several reasons.
First, you need a lot of starting ingredients. The building blocks that make up proteins — small molecules called amino acids — come in 21 different types. To get a working protein, the right combo must be linked up in the right order, then folded just so.
Another problem: Proteins are fragile. Changes in pH and temperature quickly break them. Often permanently. That makes working with proteins finicky and costly.
Biologists like Rawson and Göpfrich found a workaround. They’re creating nanomachines that do the same jobs as proteins. And with the right design, these nanomachines can do so easier and at less cost. They also can be more stable — and use fewer ingredients.
To build them, researchers can work with many types of materials. Some use metals, like those in big machines. Rawson uses carbon nanotubes. Göpfrich and some others even use biological molecules, such as DNA.
DNA origami
Like proteins, DNA forms long, threadlike molecules that can self-fold into 3-D shapes. Scientists have made protein-mimicking molecular machines out of DNA. This field — called DNA origami — started in 2006 when biologist Paul Rothemund created DNA that self-folded into tiny smiley faces, as shown here.

DNA withstands temperature and pH changes better than lab-made proteins. It’s also simpler to make, needing only four types of building blocks (not proteins’ 21 amino acids).
Ushering in synthetic biology
Such scientists are part of a field known as synthetic biology. That’s the study of human-made or modified life. Michael Levin is a synthetic biologist at Tufts University in Medford, Mass. In a 2022 podcast, he described one of his grand-scale visions for the field: Rather than try to heal old or damaged body parts, he’d replace them with new ones.
Imagine you could make a new organ or body part, he said. Maybe it’s a new lung for someone whose breathing tissue is riddled with cancer. Or maybe engineers would grow a replacement leg for an amputee. You might even design a novel animal.
“Say that you want a six-legged frog with a propeller at the top,” Levin said in his podcast. “If we really knew what we were doing,” synthetic biologists could direct cells to build it.
Such technology is already underway, Levin said, with the goal of repairing injuries, eliminating aging-related problems and more. “It would fix everything except infectious disease.” It’s a distant goal, he admitted — in fact, one he likely won’t live to see. But he believes other generations will: “I think it’s achievable.”
Can synthetic machinery fix broken cells?
A fully synthetic animal appears to be far off. Scientists have, however, learned to make small synthetic-biology fixes. Our cells and molecules must work together. Think of them like “the wiring of a house,” Rawson says. Flip a switch, and a light turns on. That’s a big-scale effect. But each small piece in the system must work properly to get that end effect.
Biological systems are similar. Their many small parts must work as teams.
Here’s one example. Cells in our eyes contain a blob-shaped protein called rhodopsin (Roh-DOP-sin). When light strikes it, this protein splits apart. That triggers a message that zips through wire-like nerves to our brain and tells us what we’re looking at.
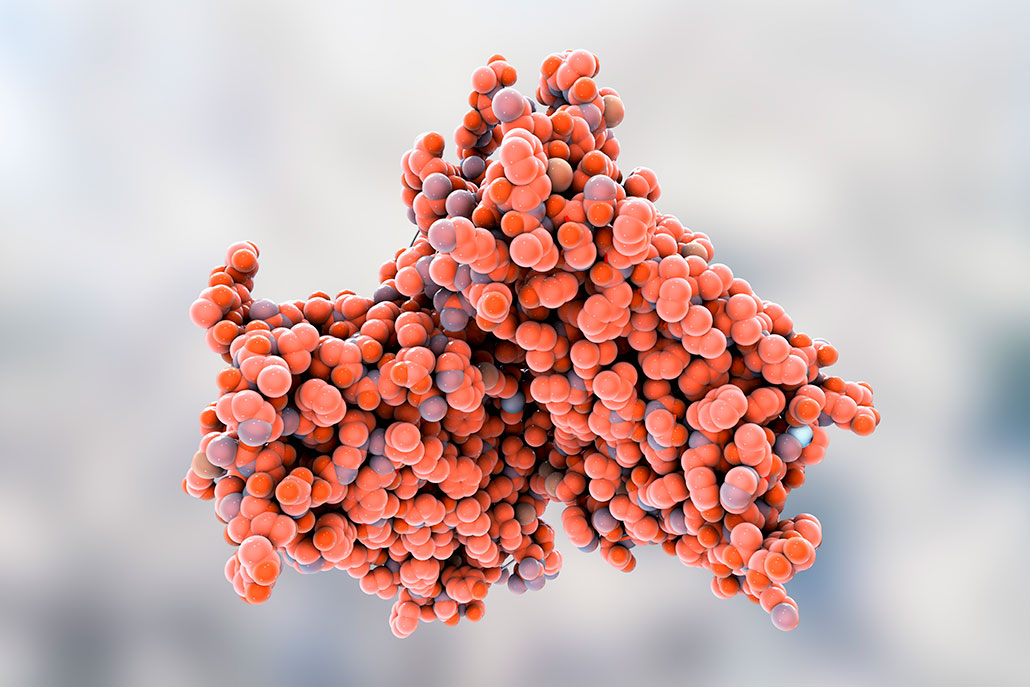
Splitting that rhodopsin is like flipping a light switch.
If that light switch breaks, however, the lights go out. Similarly, if the rhodopsin pathway fails, we cannot see. But synthetic biology, Rawson says, can help replace a faulty switch or re-wire the system.
It might tackle the eye disease retinitis pigmentosa (Reh-tih-NY-tis Pig-men-TOH-suh). It develops when cells don’t make rhodopsin properly.
Scientists have already inserted light-sensitive nanoparticles into the eyes of rats with the disease. The nano bits contain a molecule used in solar panels (called P3HT). As the nanoparticles link up, they form light-sensitive, nerve-like wiring. Afterward, the rats could see.
Researchers shared these findings in the June 2022 Nature Communications.
Leeches stand in as lab ‘rats’
Researchers are now looking for tech solutions to broken “wiring” in people. For instance, we already have mind-controlled prosthetic limbs. Both vision-restoring bionic eyes and brain chips seem ripped straight out of science fiction. Each can replace some damage to the body’s signaling networks. But they all require electronics to communicate with our brains.
And such implants carry risks, says Hanne Biesmans. She studies bioelectronics at Linköping University in Stockholm, Sweden. The problem: Electrodes — usually metal wires — must protrude out from some implant and into the brain. Those electrodes may damage delicate, squishy tissue around them.
Biesmans’ lab is working on a new tactic: growing synthetic nerves inside the body. No more metal wires. Her team’s gentle pathways will use the same carbon-based chemistry found in our bodies.
As a test, Biesmans’ team created the new nerve-like networks inside living leeches. Then they used those networks to make the leeches move.
To do this, they injected leeches with a mix of chemicals. The mix contained small molecules that can snap together to form long conductive “wires.” Next, they added chemicals that trigger those molecules to link up into “nerves.” (Our bodies use a similar technique to build large proteins from smaller amino-acid building blocks.)
In tests, a gentle electrical pulse to the synthetic nerves triggered muscle contractions in the leeches — much as a real nerve would.
Biesmans’ team shared these findings last February in Science.
The group hopes such synthetic nerves might one day provide a gentler alternative to today’s deeply penetrating electrodes. They haven’t yet tried their new technology on brain tissue. But such work opens the door for a new generation of computer interfaces with the brain.
Will synthetic cells mimic real ones?
These approaches start with a living cell or organism, then modify it. Göpfrich uses a different strategy: Start with chemicals and other building blocks to create something life-like.
She starts with a somewhat cell-like goop, such as a bubble of oil. Then she equips that bubble with tiny machines that grant it life-like traits. Her goal, she says, is to “create life from scratch.”
Göpfrich sometimes draws inspiration from real cells. For instance, cells build internal transport systems. These use a type of protein called a microtubule. Such proteins work like a toy train set, with each microtubule like a piece of track. They can link to form paths throughout a cell. Other proteins can then move along these rails to move food, wastes and other things from one side of a cell to another.
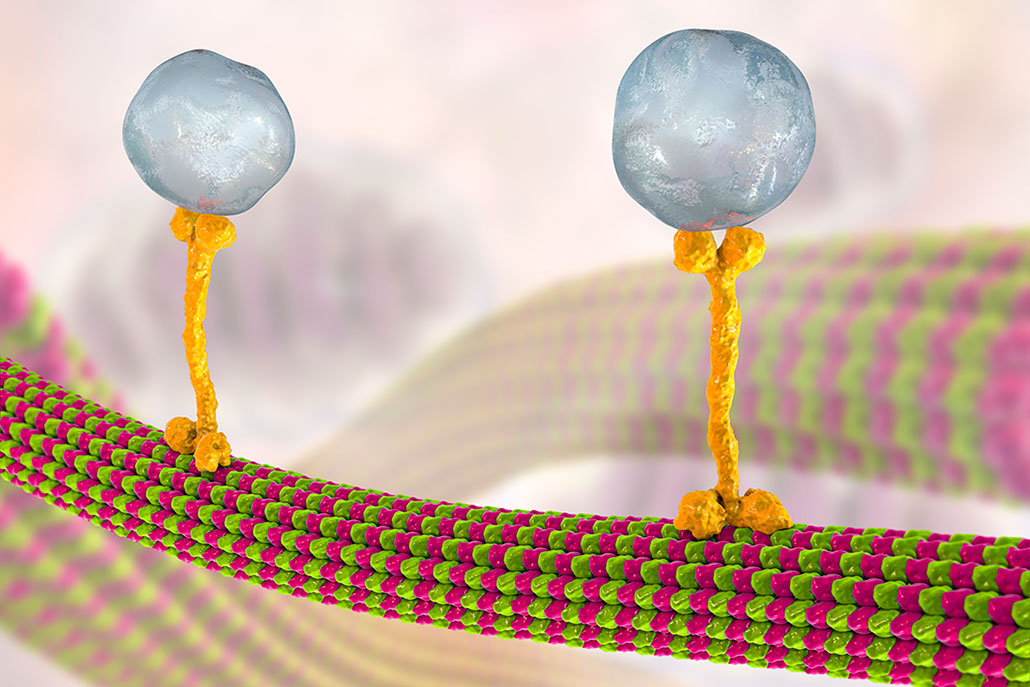
Göpfrich and her team has now created DNA-based nano-machines to do much the same thing. Like microtubules, they connect and form tracks. And these nano-tracks can dissolve and reform in response to different signals, such as light or the presence of some other chemical.
In one test, these tracks moved tiny nano-bundles of gold. The researchers described the work in June 2022 in Nature Chemistry.
Göpfrich is also interested in mimicking a very basic role of cells: reproduction.
To reproduce, cells first duplicate everything inside them. After sending half the contents to each side, the cell then pinches in half. Many of these steps use those railroad-like microtubules to move materials.
Göpfrich wanted her artificial cells to divide like living ones do. So she started with little oil bubbles. They’d serve as empty cells. Then her team inserted DNA nanoparticles into each bubble. They used two types of these particles, putting one type on each side of the cell.
When triggered by light, each type of particle allowed different amounts of water to enter its side of the cell. Those differences made this synthetic cell split in two. And each of the new ones could later divide again and again.
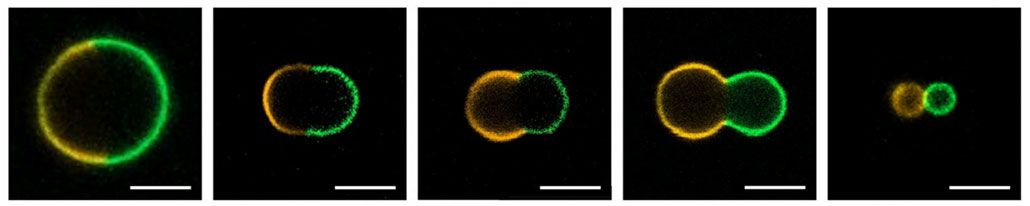
Can we give cells new abilities?
Rawson at the University of Nottingham is using synthetic biology to give cells all-new jobs. In one project, he inserted synthetic machinery into living cells. These nano-machines use carbon nanotubes. Treated cells can now sense electrical fields.
The tubes mimic normal, straw-like proteins in cell membranes. Called channel proteins, these gated structures can open to let chemicals pass through — or close to keep them out. Through such channels, cells slurp water and nutrients. They’re important because they control what enters and leaves cells.
The signal to open and close differs from one channel to another. Channels in our nerves, for example, open in response to chemical signals.
Rawson used nanotubes to make channels that respond to other signals. Some flip open in response to a gentle electric current. Added to living cells, these nanotubes can make the cells respond to a signal it normally would ignore (here, electricity). With the press of a button, Rawson can open pores in a cell’s membrane. That allows him to send some chemical inside.
Published in the July 16, 2021, issue of Small, these findings suggest people now can modify cells to sense things they couldn’t before.
Other scientists have created synthetic cells that will open channels in response to magnetic fields, sound waves and even beams of light.
Arming cells to fight disease
Such superpowers now allow scientists to control the behavior of cells in new ways.
In 2023, for instance, Rawson used his nanotubes to target and destroy cancer cells.
Carbon nanotubes naturally pass into cell membranes. When added to a mix of healthy cells and cancer cells, they seem to prefer the cancer cells, Rawson finds. Once they’re in place, he turns on an electrical current. The nanotubes respond by flipping from sideways to upright in the membrane, opening a new channel into the cell. That sets off a chain reaction that directs the cancer cells to self-destruct.
Rawson’s group shared its findings last September in Nature Nanotechnology.
Nicholas Leeper also uses carbon nanotubes to make membrane channels. But rather than target cancer cells, his target cells of the immune system.
Working at Stanford University’s School of Medicine in Palo Alto, Calif., this doctor uses those synthetic channels to boost the defenses of healthy cells. Leeper studies things that cause heart disease. These include plaque build-ups that can clog blood vessels and arteries. Leeper is trying to supercharge immune cells so that they will chow down on that plaque.
Their newly assigned task is to “gobble up diseased tissue.” These cells pull the nanotubes into their outer membranes. “We don’t know why,” says Leeper, but “most other cells in the body will ignore them.” That means his team can sneak something into the immune cells without bothering other cells.
Leeper then adds a drug that makes the altered cells very hungry. Once they reach an artery inflamed with plaque, they gobble up the cholesterol and sick cells that make up this plaque.
Basically, they help “take out the garbage,” he says.
Leeper’s team has already used this technique to halt the buildup of plaque in the arteries of mice with high cholesterol. They reported details of their findings in January 2020.
Ultimately, Göpfrich says, altering or creating life “from scratch” is driven by curiosity about what’s possible. Work by her group and other synthetic biologists is giving rise to new tools and ideas.
And with those tools, scientists are starting to dream beyond what simply exists in nature.





