Making caffeine content crystal clear
This young teen crystalized the popular pick-me-up right out of a soft drink
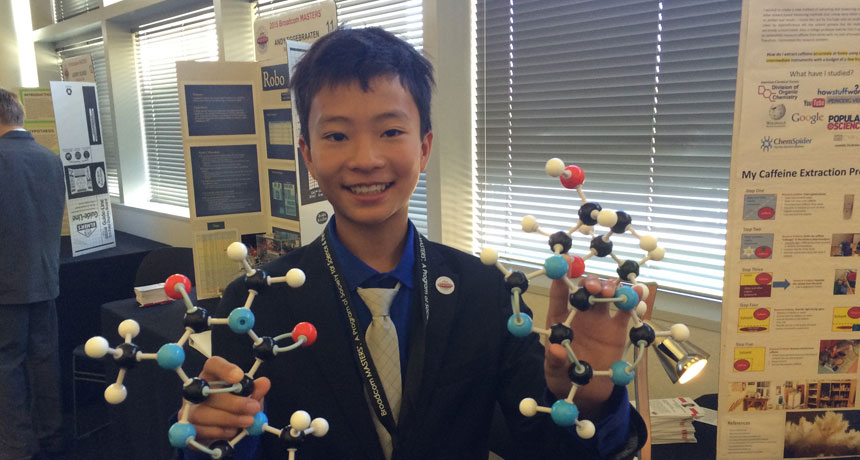
Maximillian Du shows off chemical models of caffeine and adenosine. The teen set out to measure how much caffeine was in 10 different drinks.
B. Brookshire/SSP
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
SAN JOSE, Calif.— Some people might get a chemistry set for Christmas and play with it once or twice. But for Maximillian Du, 13, the holiday present sparked an obsession. It became the basis of his own chemistry lab and his latest project — creating a new method to measure caffeine in everything from coffee to soda pop.
“My mom has a problem,” explains Max, now in eighth grade at Eagle Hill Middle School in Manlius, N.Y. “She can stay up all night if she drinks a cup of coffee. But she can go to sleep with a cup of tea.” This is likely due to the different amounts of caffeine and other such stimulants in the beverages. Green plants make caffeine, probably to deter pests such as insects from dining on their leaves. But in people, this chemical acts as a stimulant. It blocks the action of adenosine, a natural chemical that makes us feel sleepy. When adenosine cannot act, we feel more alert.
Max decided to measure just how much caffeine was in 10 different drinks. Among them were instant coffee, tea, energy drinks and soft drinks. He used decaffeinated coffee and grape juice as controls (allowing him to compare drinks with caffeine against drinks without caffeine). Many companies measure caffeine in their beverages. They use a method called ultraviolet spectroscopy, Max explains. It measures how much ultraviolet light — light close to violet, but wavelengths that people cannot see — is absorbed by different chemicals. It is a very accurate method, but also too expensive for this teen.
So Max decided to extract caffeine using a chemical method. He says that “it’s an easy activity for people to do.”
The teen went online and found out that the chemical ethyl acetate might help. It’s a solvent — a material that can help other materials dissolve into a solution. He soon found that adding this sweet-smelling, colorless liquid to the drinks worked. It caused caffeine to move from the beverage into the ethyl acetate. To boost the speed of that reaction, he added sodium hydroxide to each beverage. It makes the drinks more alkaline. (This chemical is commonly used to make things such as soap and drain cleaners.)
But it wasn’t enough to move the caffeine into the ethyl acetate and some water. To measure the caffeine, he wanted collect it as a dry powder. So Max added heat until the ethyl acetate boiled off. Traces of water remained, so the teen added magnesium sulfate and calcium chloride. The two chemicals, which are very attracted to water, dried out his samples. At last he had pure caffeine crystals, which he could now weigh.
Max showed off those crystals at a competition known as Broadcom MASTERS (for Math, Applied Science, Technology and Engineering for Rising Stars). This science program was created by Society for Science & the Public. It is sponsored by Broadcom, a company that builds devices to help computers connect to the Internet. The annual event brings together middle school students with winning science-fair projects from across the United States. Finalists shared their work with each other and the public in San Jose, Calif., on October 3.

Max wanted to see if the amount of caffeine that beverage companies claim on a product’s label matches what is actually in them. And for canned or bottled drinks, he found, the amounts “are pretty close” to what’s listed on a label. But when a drink is brewed at home, he found that the values “are way off.” A drinker determines how long she keeps her tea bag in the hot water, or how many coffee beans she grinds up for her coffee. Coffee brewed from a large heap of beans and not much water will have a lot more caffeine than when it’s made with few beans and a lot of water.
In the future, Max wants to extract caffeine using fewer materials. This should make his process less costly. But he does warn future chemists that by the time they’re done, there’s not going to be any beverage left to enjoy. He explains that “you can’t test the caffeine in your Coke and then drink your Coke.” The process that takes the caffeine out also adds chemicals you won’t (and shouldn’t) drink. For instance, he notes, the sodium hydroxide he added “is toxic and it also tastes horrible.” So while his caffeine extraction was fun, he says that if you want to avoid caffeine in your drinks, it’s probably best to just buy the decaffeinated kind.






