When love-hate is materially good
Researchers copy nature's water-loving, water-repelling ways to make smarter stuff
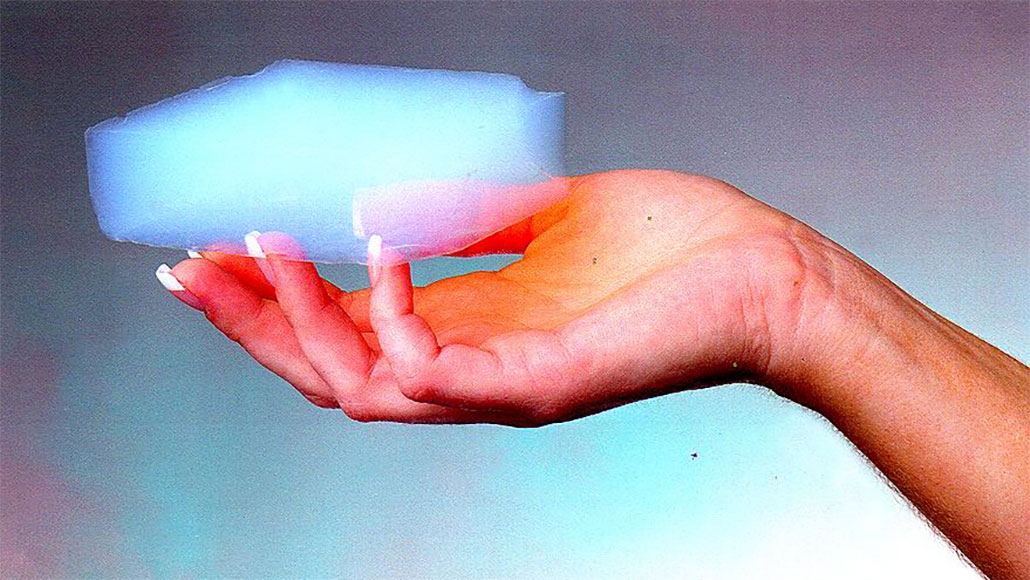
An aerogel, shown in someone's hand, is a silica material littered with so many tiny holes that it's about 99 percent air and 1 percent silica. New research adds a twist to this property.
NASA, JPL, Caltech
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
Scientists are copy cats: They get some of their best ideas by cribbing from nature. After all, with hundreds of millions of years of evolution under its belt, nature has come up with some pretty wonderful stunts. Think of geckos walking up glass walls or water striders skating on ponds.
One chemical engineer who has tremendous respect for nature is C. Jeffrey Brinker. “When I was very young, I got a microscope and loved looking at stuff from the outside,” he says. “I was totally fascinated watching protozoa and things I found under rocks.” Protozoa are small, one-celled animals.
Tacked to the ceiling in Brinker’s office is a tumbleweed that blew across the high desert of Albuquerque, N.M., where he lives.
What intrigued Brinker about the tumbleweed was its repeated branching and open structure ― how the largest branches give way to smaller branches. “I was always struck by how few tumbleweeds you can fit in a trash bag,” he says. “They don’t easily slip into each other. They stay open and porous. I wanted to emulate those properties.”
Brinker hoped to make materials that would have the same open structure, but at a much, much smaller size. He envisioned that the pores — holes full of air ― in the materials would be only a few nanometers wide. (A nanometer is a billionth of a meter. There are about 100,000 nanometers in the width of a hair. In the time it takes you to read this sentence, your fingernails will have grown 1 nanometer.) An extra-porous material could find all kinds of applications such as being used as an extremely strong insulator, or nosing around for toxic chemicals in the air.
One very open, very porous material is called an aerogel. Some aerogels are basically 99 percent air and 1 percent silica (silicon dioxide). While an aerogel starts out wet and squishy like Jello, as it loses water it ends up stiff, light and dry. In your hand, a dried-out aerogel feels like a hard, weightless lump of smoke.
Because it is mostly air, an aerogel is a very good insulator: It doesn’t let heat and electricity travel through. NASA, for example, uses aerogels to protect the Mars Exploration Rovers from extreme cold and heat.
Brinker has discovered a safe, adaptable way to make an aerogel. And he did it by looking, of course, to nature.
Collect water like a bumpy beetle
To understand what Brinker was thinking, go to one of the most desolate, driest places on the planet. The Namib Desert in southwest Africa gets only a minuscule bit of rain. Yet some creatures manage to thrive there.
Sit on a dune in the evening or early morning, and you might see a perfectly healthy black Namib Desert beetle crawl out from the sand and do a curious thing: stick its butt up in the air.
When you stop giggling, you might notice that the beetle’s back is bumpy. If you were very observant, you’d start to see tiny drops of water growing on the beetle’s bumps. When these drops got big enough they would roll off the bump and sluice down the surrounding channels like a bobsled, to be delivered to the beetle’s mouth.
Even in one of Earth’s driest places, a faint fog appears in the evening and early morning. The beetle is able to collect extremely small water droplets from these mists because the bumps and channels on its back are made of materials whose properties are called “hydrophilic” and “hydrophobic.”
“Hydro” means water. “Philic” means love, and “phobic” denotes fear. So a material that is hydrophilic is attracted to water, whereas a hydrophobic material, like wax, repels water.
The beetle’s hydrophilic bumps attract water droplets until the droplets grow big and heavy enough to speed down the hydrophobic troughs.
Hydrophobic and hydrophilic are responsible for a lot of nature’s wonders, such as the water-walking water strider and the self-cleaning lotus plant. In fact, without hydrophobic and hydrophilic, you would not be here at all.

Scientists think that the very first cells could have been created when molecules with hydrophilic heads and hydrophobic tails arranged themselves into a hollow sphere. These “amphiphilic” or “amphipathic” molecules (“amphi” means “both”) formed the first cell membranes by clustering their tails inside the ball and facing their heads out toward the surrounding water.
Like a cowboy wagon round-up, the amphiphilic molecules surround and shield the inner part of the sphere from the outside world. For a cell, this means that the living machinery inside is protected, with the cell membrane in charge of keeping out harmful molecules but letting nutrients in and wastes out.
Silica fossil skeleton
Back in New Mexico at the University of New Mexico and at Sandia National Laboratories where Brinker works, hydrophobic and hydrophilic gave him an idea. The silica used in aerogels is hydrophilic because it usually has hydroxyl (one oxygen and one hydrogen) molecules on its surface. Hydroxyl groups are very reactive: They readily bond with the silica, water and each other. As the silica gel mixture dries, it shrinks and collapses in on itself due to drying stresses. Because the hydroxyl-silica groups like to bond, they stick to each other and chemically “lock in” the collapsed structure. Try the same thing at home by drying Jello in the oven. It will form hard glassy globs that are much denser than the original dessert, says Brinker.
Brinker thought that if he attached hydrophobic molecules to the silica, liquid could be extracted at room temperature and pressure without permanent shrinkage. He was right. While it dries, the silica still gets compressed like a spring, but because the hydrophobic molecules don’t bond together the silica network doesn’t stick to itself.
Brinker had another insight. He thought about the way amphiphilic molecules arrange themselves when they’re in water. He realized that he could use this behavior to corral silica into orderly patterns. Being able to manipulate silica is good because silica is plentiful, inexpensive and easy to control. It’s already used in many products including glass windows, optic fibers, microelectronics and toothpaste.
This is Brinker’s recipe:
Dissolve silica in water and mix with a kind of soap (made of amphiphilic molecules) and some alcohol. Slowly dry the mixture. As the water and alcohol evaporate, the soap molecules organize themselves into different arrangements. One pattern looks like a stack of wagon wheels. Another looks like a honeycomb in a beehive.
Because the silica is attracted to the hydrophilic part of the soap molecules, it gets dragged along with the soap and pushed into the wagon-wheel and honeycomb patterns. When the liquids have evaporated, Brinker removes the soap, and what’s left is a kind of airy, patterned “fossil skeleton” made of silica.
“Jeff’s work is pioneering”, says Hugh W. Hillhouse, a chemical engineer the University of Washington in Seattle. “These types of [materials] are playing a significant role in the development of new technology,” including sensors to detect explosives or to monitor drinking water, innovative ways of making solar cells, and the delivery of drugs targeted at cancer cells.
It turns out that the open, ordered patterns in a thin film of silica make for an extremely hydrophobic material, one that scientists call “superhydrophobic.” When the film is put on a surface, a drop of water bounces off it like a rubber ball. Brinker has also a made a surface that mimics the Namib beetle’s back.
Houses for cells
Brinker’s group has gone one step further. The researchers have been able to add all kinds of things to the silica recipe: Chemicals that turn bright blue under ultraviolet light. Or molecules that open and close, acting like gates to the tiny pores in the silica skeleton. The scientists combined materials that normally don’t mix well to make a tough and resilient kind of mother-of-pearl, modeled after that found in shells.

The most astounding addition, though, has been living cells.
Each cell (such as a yeast cell or even a bacterium) actually helps organize the silica network around itself like armor, trapping water into the pore with it as it holes up into its tiny apartment. So even as the material gets dried out, a cell keeps water safe in its one pore.
Some of the cells can live this way for a year. The researchers have put some on several Space Shuttle missions, and the cells have survived the ride. Other cells survived a very hot Albuquerque summer afternoon in the car trunk of a graduate student who had forgotten about them.
Because it can house long-lived cells, this silica network is a biologist’s dream. For the first time, scientists can zero in on the workings of an individual cell in a controlled environment that is similar to the environments cells inhabit in living beings. Already Brinker’s lab has discovered something important about bacteria cells and possibly cancer.
What do you think Jeff Brinker’s next idea will be? Whatever it is, chances are he’ll get some of his inspiration from Mother Nature.
SIDE STORY
Target: Bacteria, and maybe cancer cells someday
With the help of their nature-inspired silica material, Jeff Brinker and his co-workers at the University of New Mexico have homed in on bacteria cells that cause staph infections. These infections can be deadly, and the widespread use of antibiotics has made some staph-causing bacteria resistant to drugs.
Scientists had thought that these bacteria become harmful only after gathering in large groups and then beginning to signal each other, by emitting “alarm” molecules, to make toxins. By isolating individual bacteria within “igloos” in the new material they developed, however, Brinker’s team discovered that an individual bacterium sends out alarm molecules ― even when no other bacteria are around. What’s more, the team discovered for the first time that a bacterium’s own alarm molecules can induce it to emit toxins.
This means that the alarm system probably originally evolved not so much to signal other bacteria, but to act as a kind of radar to sense a bacterium’s environment, says Brinker’s co-worker Eric Carnes, a lecturer in the UNM chemical and nuclear engineering department.
When your body attacks bacteria, it launches an army of immune cells. One kind of immune cell swallows and squishes the offending microbe in a pouch before it bombards it with poisons. In response, the bacterium emits alarm molecules. When these bounce back from the enclosed space and hook on to the bacterium’s surface, the bacterium spews out toxins with a vengeance.
In the researchers’ experiment, the cramped confines of the silica enclosure mimic an engulfing immune cell, making the bacterium think it’s being attacked.
“When something’s trying to kill us, we take it personally,” say Carnes. “A bacterium isn’t inherently malicious. It was just hanging out in the body, but when something attacks it, of course it’s going to freak out and fight back, dumping out toxins and sounding the alarm, ‘This thing’s going to eat me!’”
Now Brinker’s team is looking for ways to stop the alarm molecules from getting into each bacterium’s mailbox. Introducing another kind of molecule, one that clings to the alarm molecules, kept the alarm molecules from attaching to the staph-causing bacteria. Ultimately, this trick could provide a way to conquer only staph-causing bacteria without hurting the other, good bacteria that live in the digestive tract.
Brinker thinks the bacteria study might provide clues to understanding cancer cells. Cancer that starts in one part of the body becomes particularly lethal when it moves to another part. This is called metastasis. Some scientists think that cancer cells signal one another just like bacteria do.
Using the same kinds of silica houses, Brinker’s lab is exploring which conditions make a cancer cell become inactive and which make it metastatic and aggressive. This understanding will help scientists figure out how to better target the dangerous cells.
To single out and kill these cells, Brinker’s group is also investigating the use of small particles made of the porous silica material. On the outside of the particles are molecules that link to cancer cells only. On the inside is a full cargo of poison to deliver to just those cells, so that the rest of the body doesn’t get sick. The chemotherapy used today to kill cancer cells also attacks healthy cells in the body, leaving patients feeling very sick.
This kind of targeted approach is going to vastly improve medicine, says Carnes. “Kids today are very lucky to get to witness this in their lifetimes.”





