Explainer: How batteries and capacitors differ
Each energy-storage device has its own advantages and disadvantages
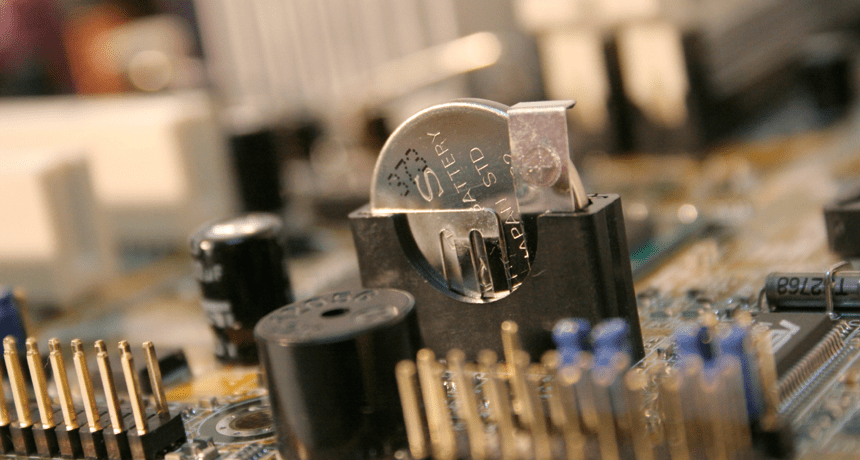
Many electronic circuits (like the one shown) are powered by batteries. Increasingly, however, engineers are looking to capacitors as another option for providing energy as needed to all or parts of such circuits.
fotek/iStockphoto
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
By Sid Perkins
Energy can be stored in a variety of ways. When you pull back on a slingshot, energy from your muscles is stored in its elastic bands. When you wind up a toy, energy gets stored in its spring. Water held behind a dam is, in a sense, stored energy. As that water flows downhill, it can power a water wheel. Or, it can move through a turbine to generate electricity.
When it comes to circuits and electronic devices, energy is typically stored in one of two places. The first, a battery, stores energy in chemicals. Capacitors are a less common (and probably less familiar) alternative. They store energy in an electric field.
In either case, the stored energy creates an electric potential. (One common name for that potential is voltage.) Electric potential, as the name might suggest, can drive a flow of electrons. Such a flow is called an electric current. That current can be used to power electrical components within a circuit.
These circuits are found in a growing variety of everyday things, from smartphones to cars to toys. Engineers choose to use a battery or capacitor based on the circuit they’re designing and what they want that item to do. They may even use a combination of batteries and capacitors. The devices are not totally interchangeable, however. Here’s why.
Batteries
Batteries come in many different sizes. Some of the tiniest power small devices like hearing aids. Slightly larger ones go into watches and calculators. Still larger ones run flashlights, laptops and vehicles. Some, such as those used in smartphones, are specially designed to fit into only one specific device. Others, like AAA and 9-volt batteries, can power any of a broad variety of items. Some batteries are designed to be discarded the first time they lose power. Others are rechargeable and can discharge many, many times.

A typical battery consists of a case and three main components. Two are electrodes. The third is an electrolyte. This is a gooey paste or liquid that fills the gap between the electrodes.
The electrolyte can be made from a variety of substances. But whatever its recipe, that substance must be able to conduct ions — charged atoms or molecules — without allowing electrons to pass. That forces electrons to leave the battery via terminals that connect the electrodes to a circuit.
When the circuit isn’t turned on, the electrons can’t move. This keeps chemical reactions from taking place on the electrodes. That, in turn, enables energy to be stored until it is needed.
The battery’s negative electrode is called the anode (ANN-ode). When a battery is connected into a live circuit (one that has been turned on), chemical reactions take place on the anode’s surface. In those reactions, neutral metal atoms give up one or more electrons. That turns them into positively charged atoms, or ions. Electrons flow out of the battery to do their work in the circuit. Meanwhile, the metal ions flow through the electrolyte to the positive electrode, called a cathode (KATH-ode). At the cathode, metal ions gain electrons as they flow back into the battery. This allows the metal ions to become electrically neutral (uncharged) atoms once again.
The anode and cathode are usually made of different materials. Typically, the anode contains a material that gives up electrons very easily, such as lithium. Graphite, a form of carbon, holds onto electrons very strongly. This makes it a good material for a cathode. Why? The bigger the difference in the electron-gripping behavior between a battery’s anode and cathode, the more energy a battery can hold (and later share).
As smaller and smaller products have evolved, engineers have sought to make smaller, yet still powerful batteries. And that has meant packing more energy into smaller spaces. One measure of this trend is energy density. That’s calculated by dividing the amount of energy stored in the battery by the battery’s volume. A battery with high energy density helps to make electronic devices lighter and easier to carry. It also helps them last longer on a single charge.

In some cases, however, high energy density can also make devices more dangerous. News reports have highlighted a few examples. Some smartphones, for instance, have caught fire. On occasion, electronic cigarettes have blown up. Exploding batteries have been behind many of these events. Most batteries are perfectly safe. But sometimes there may be internal defects that cause energy to be released explosively inside the battery. The same destructive results can occur if a battery is overcharged. This is why engineers must be careful to design circuits that protect batteries. In particular, batteries must operate only within the range of voltages and currents for which they have been designed.
Over time, batteries can lose their ability to hold a charge. This happens even with some rechargeable batteries. Researchers are always looking for new designs to address this problem. But once a battery can’t be used, people usually discard it and buy a new one. Because some batteries contain chemicals that aren’t eco-friendly, they must be recycled. This is one reasons engineers have been looking for other ways to store energy. In many cases, they’ve begun looking at capacitors.
Capacitors
Capacitors can serve a variety of functions. In a circuit, they can block the flow of direct current (a one-directional flow of electrons) but allow alternating current to pass. (Alternating currents, like those obtained from household electrical outlets, reverse direction many times each second.) In certain circuits, capacitors help tune a radio to a particular frequency. But more and more, engineers are also looking to use capacitors to store energy.
Capacitors have a pretty basic design. The simplest ones are made from two components that can conduct electricity, which we’ll call the conductors. A gap that doesn’t conduct electricity usually separates these conductors. When connected to a live circuit, electrons flow in and out of the capacitor. Those electrons, which have a negative charge, are stored on one of the capacitor’s conductors. Electrons won’t flow across the gap between them. Still, the electric charge that builds up on one side of the gap affects the charge on the other side. Yet throughout, a capacitor remains electrically neutral. In other words, the conductors on each side of the gap develop equal but opposite charges (negative or positive).
The amount of energy a capacitor can store depends on several factors. The larger the surface of each conductor, the more charge it can store. Also, the better the insulator in the gap between the two conductors, the more charge that can be stored.
In some early capacitor designs, the conductors were metal plates or disks separated by nothing but air. But those early designs couldn’t hold as much energy as engineers would have liked. In later designs, they began to add non-conducting materials in the gap between the conducting plates. Early examples of those materials included glass or paper. Sometimes a mineral known as mica (MY-kah) was used. Today, designers may choose ceramics or plastics as their nonconductors.
Advantages and disadvantages
A battery can store thousands of times more energy than a capacitor having the same volume. Batteries also can supply that energy in a steady, dependable stream. But sometimes they can’t provide energy as quickly as it is needed.
Take, for example, the flashbulb in a camera. It needs a lot of energy in a very short time to make a bright flash of light. So instead of a battery, the circuit in a flash attachment uses a capacitor to store energy. That capacitor gets its energy from batteries in a slow but steady flow. When the capacitor is fully charged, the flashbulb’s “ready” light comes on. When a picture is taken, that capacitor releases its energy quickly. Then, the capacitor begins to charge up again.
Since capacitors store their energy as an electric field rather than in chemicals that undergo reactions, they can be recharged over and over again. They don’t lose the capacity to hold a charge as batteries tend to do. Also, the materials used to make a simple capacitor usually aren’t toxic. That means most capacitors can be tossed into the trash when the devices they power are discarded.
The hybrid
In recent years, engineers have come up with a component called a supercapacitor. It’s not merely some capacitor that is really, really good. Rather, it’s sort of some hybrid of capacitor and battery.
So, how does a supercapacitor differ from a battery? The supercapacitor has two conducting surfaces, like a capacitor. They’re called electrodes, as in batteries. But unlike a battery, the supercapacitor stores energy on the surface of each of these electrodes (as a capacitor would), not in chemicals.
Meanwhile, a capacitor normally has a non-conducting gap between two conductors. In a supercapacitor, this gap is filled with an electrolyte. That would be similar to the gap between the electrodes in a battery.
Supercapacitors can store more energy than regular capacitors. Why? Their electrodes have a very large surface area. (And the larger the surface area, the more electrical charge they can hold.) Engineers create a large surface area by coating the electrode with a very large number of very tiny particles. Together, the particles produce a rugged surface that has much more area than a flat plate would. That lets this surface store far more energy than a regular capacitor can. Still, supercapacitors can’t match the energy density of a battery.
CORRECTION: This story has been revised to correct one sentence that had inadvertently switched the term cathode for anode. The story now reads correctly.







