Explainer: What are the different states of matter?
Most people know solids, liquids and gases — but what about the four others?

A geyser in Iceland exhibits the three main states of matter of H2O. As a liquid, H2O is known as water. The steam coming off the geyser is the gas form of H2O, and the surrounding snow shows H2O in its solid state.
John and Tina Reid/Getty Images
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
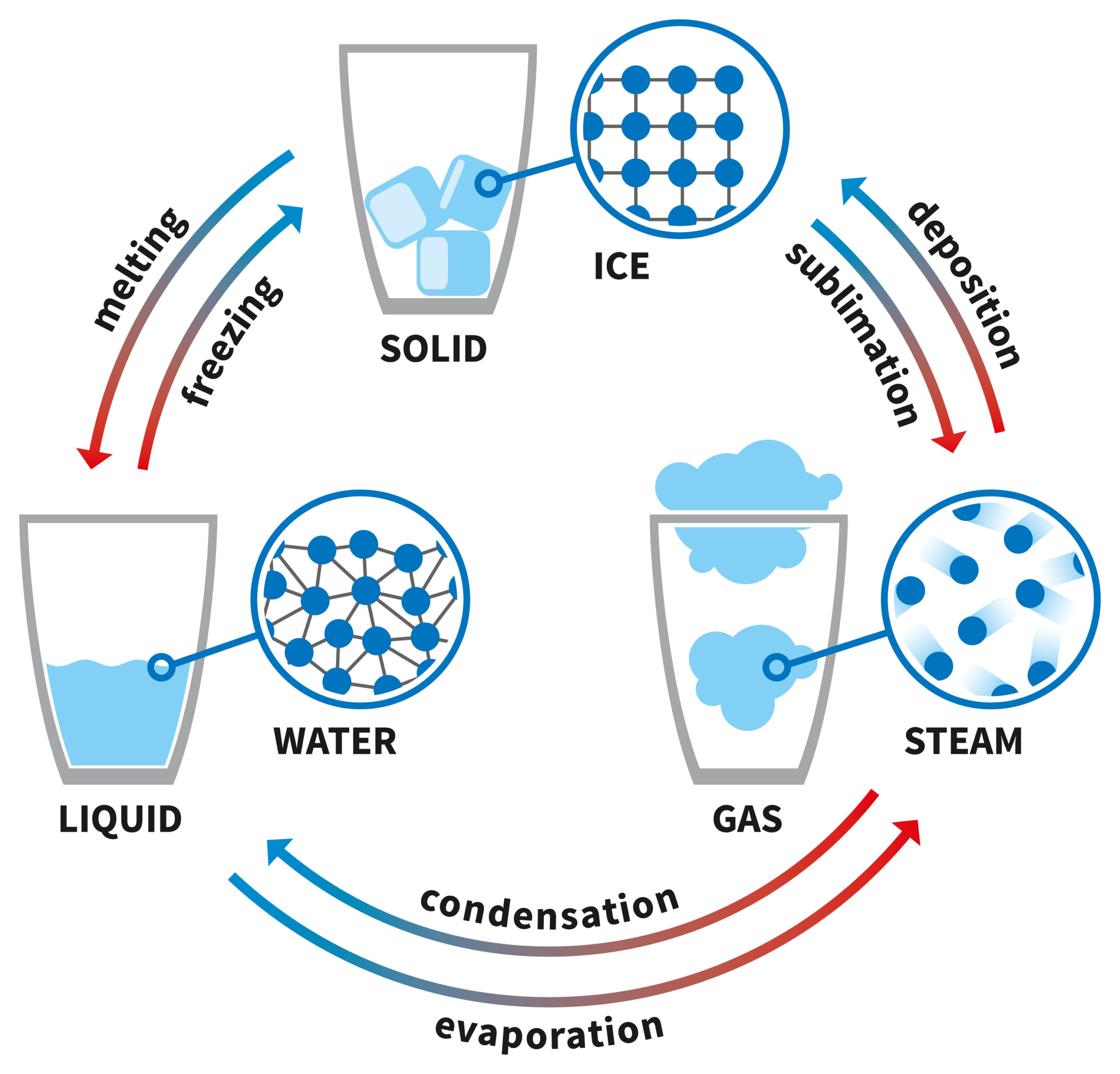
Ice, water and vapor are three distinctly different forms — or states — of water. Like other substances, water can take different forms as its surrounding environment changes. Take, for example, an ice-cube tray. Pour water into the tray, stick it in the freezer and a few hours later that liquid water will have transformed into solid ice. The substance in the tray is still the same chemical — H2O; only its state has changed.
Put the ice into a pot over a flame on the stove and it will melt back down to liquid. If it gets hot enough, you will notice steam rising off of the liquid. This vapor is still H2O, just in gas form. Solid (the ice), liquid (the water) and gas (the vapor) are the three most common states of matter — at least on Earth.
In ancient Greece, one philosopher recognized how water could change form and reasoned that everything must be made of water. However, water isn’t the only type of matter that changes states as it’s heated, cooled or compressed. All matter is made of atoms and/or molecules. When these tiny building blocks of matter change their structure, their state or phase does too.
Solid, liquid and gas are the best-known states of matter. But they’re not the only ones. Lesser-known states develop under more extreme conditions — some of which never exist naturally on Earth. (They can only be created by scientists in a laboratory.) Even today, researchers are still discovering new states of matter.
While there’s likely more awaiting discovery, below are seven of the currently agreed-upon states that matter can take.
Solid: Materials in this state have a definite volume and shape. That is, they take up a set amount of space. And they’ll maintain their shape without the help of a container. A desk, phone and tree are all examples of matter in its solid form.
The atoms and molecules that make up a solid are tightly packed together. They’re so tightly bound that they do not move freely. A solid may melt into a liquid. Or it might sublimate — turn directly from solid to gas when brought to certain temperatures or pressures.
Liquid: Materials in this state have a definite volume but no defined shape. Squeezing a liquid will not compress it into a smaller volume. A liquid will take the shape of any container into which it’s poured. But it will not expand to fill the entire container holding it. Water, shampoo and milk are all examples of liquids.
Compared to the atoms and molecules in a solid, those in a liquid are usually less tightly packed together. A liquid could be cooled into a solid. When heated enough, it will usually become a gas.
Within the most common phases of matter, other states may appear. For example, there are liquid crystals. They appear to be a liquid and flow like a liquid. Their molecular structure, however, better resembles solid crystals. Soapy water is an example of a common liquid crystal. Many devices make use of liquid crystals, including cell phones, TVs and digital clocks.
Gas: Materials in this phase have no definite volume nor shape. A gas will both take the shape of its container and expand to fill that container. Examples of common gases include helium (used to make balloons float), the air we breathe and the natural gas used to power many kitchen ranges.
The atoms and molecules of a gas also move more rapidly and freely than those in a solid or liquid. The chemical bonds between the molecules in a gas are very weak. Those atoms and molecules are also farther apart than those of the same material in its liquid or solid forms. When cooled, a gas may condense into a liquid. For instance, water vapor in air can condense outside a glass holding ice-cold water. This can create tiny water droplets. They can run down the side of the glass, forming small pools of condensation on a tabletop. (That’s one reason people use coasters for their drinks.)
The word “fluid” can refer to a liquid or a gas. Some fluids are supercritical. This is a state of matter that occurs at a critical point of temperature and pressure. At this point, liquids and gases cannot be told apart. Such supercritical fluids occur naturally in the atmospheres of Jupiter and Saturn.
Plasma: Like a gas, this state of matter has no definite shape nor volume. Unlike gases, however, plasmas can both conduct an electric current and create magnetic fields. What makes plasmas special is that they contain ions. These are atoms with an electric charge. Lightning and neon signs are two examples of partially ionized plasmas. Plasmas are often found in stars, including our sun.
A plasma can be created by heating a gas to extremely high temperatures. A plasma may also form when a jolt of high voltage moves across a space of air between two points. Though they are rare on Earth, plasmas are the most common type of matter in the universe.
Bose-Einstein condensate: A very-low-density gas that has been cooled to near absolute zero transforms into a new state of matter: a Bose-Einstein condensate. Absolute zero is thought to be the lowest temperature possible: 0 kelvin, –273 degrees Celsius or about –459.67 degrees Fahrenheit. As this low-density gas gets into such a super-cold regime, all of its atoms will eventually begin to “condense” into the same energy state. Once they reach it, they will now act as a “superatom.” A superatom is a cluster of atoms that act as if they were a single particle.
Bose-Einstein condensates don’t develop naturally. They form only under carefully controlled, extreme laboratory conditions.
Degenerate matter: This state of matter develops when a gas is super-compressed. It now begins to act more like a solid, even though it remains a gas.
Normally, atoms in a gas will move rapidly and freely. Not so in degenerate (Deh-JEN-er-ut) matter. Here, they are under such high pressure that the atoms smoosh closely together into a small space. As in a solid, they can no longer move freely.
Stars at the end of their lives, such as white dwarfs and neutron stars, contain degenerate matter. It’s what allows such stars to be so small and dense.
There are several different types of degenerate matter, including electron-degenerate matter. This form of matter contains mostly electrons. Another example is neutron-degenerate matter. That form of matter contains mostly neutrons.
Quark-gluon plasma: As its name suggests, a quark-gluon plasma is made up of the elementary particles known as quarks and gluons. Quarks come together to form particles like protons and neutrons. Gluons act as the “glue” that holds those quarks together. A quark-gluon plasma was the first form of matter to fill the universe following the Big Bang.
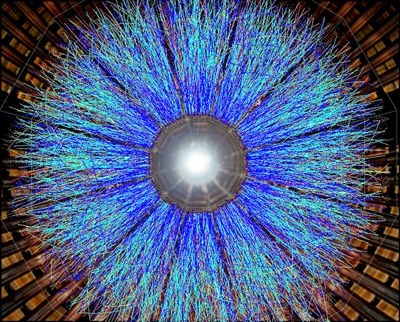
Scientists at the European Organization for Nuclear Research, or CERN, first detected a quark-gluon plasma in 2000. Then, in 2005, researchers at Brookhaven National Laboratory in Upton, N.Y., created a quark-gluon plasma by smashing gold atoms together at close to the speed of light. Such energetic collisions can produce intense temperatures — up to 250,000 times hotter than the sun’s interior. The atom smashups were hot enough to break down the protons and neutrons in the atomic nuclei into quarks and gluons.
It had been expected that this quark-gluon plasma would be a gas. But the Brookhaven experiment showed it was actually a sort of liquid. Since then, a series of experiments have shown that the plasma acts as a super-liquid, exhibiting less resistance to flow than any other substance.
A quark-gluon plasma once filled the entire universe — like a kind of soup — from which matter as we know it emerged.
And more? As with liquid crystals and supercritical fluids, there are even more states of matter than those described above. As researchers continue working to understand the world around us, they will likely keep finding newer and stranger ways that atoms, which make up everything in the world around us, behave under extreme conditions.





