Explainer: Radiation and radioactive decay
Some radioactive elements shed parts of themselves as they attempt to become nonradioactive
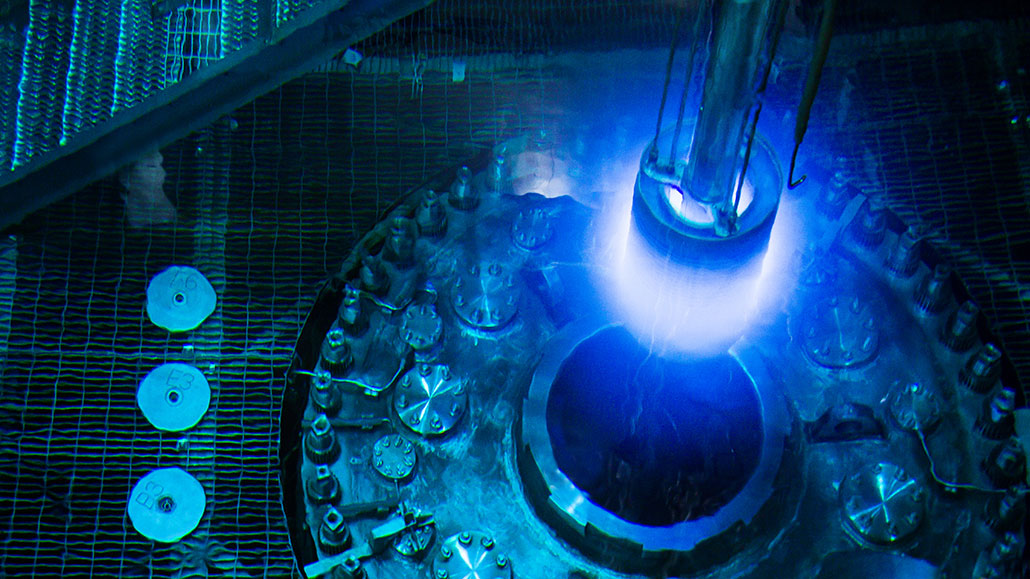
The watery core of a nuclear reactor is shown here during refueling. The blue glow comes from Cherenkov radiation. The radioactive decay of atoms in the fuel produces charged particles that move at very high speeds. These perturb atoms in the water, causing them to give off light in this distinctive hue.
Genevieve Martin/ORNL/Flickr (CC BY 2.0)
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
By Janet Raloff and Trisha Muro
Chemical elements can take several related forms, known as isotopes. Some of these forms are unstable, also known as radioactive isotopes. But they don’t want to be unstable. So they morph by shedding one or more subatomic particles. Through this process, they naturally transform into a more stable (and always smaller) element.
The expelled particles and energy are known as radiation. That morphing process is called radioactive decay.

The radiation emitted by that decay can take several forms. Often, it sheds light (a form of energy), an alpha particle (two neutrons bound to two protons) or an electron or a positron. But there are a whole host of other tiny particles that might also be shed.
You can picture the decay process by imagining a bowl filled with green and purple grapes. The bowl represents an atom’s nucleus. Each green grape represents a proton. Each purple grape stands in for a neutron. Let’s say the bowl fits exactly 40 grapes (which would represent the nucleus of a calcium atom). Now let’s imagine that you try to put in 22 purple grapes instead of 20. You might be able to balance the two extra grapes on top of the pile for a while. But sooner or later, even a small bump to the side of the bowl will make at least one of them spill out.
The protons and neutrons inside the nuclei of radioactive isotopes are unstable in a similar way. But it doesn’t take a tap to make an unstable atom decay. Forces holding together the protons and neutrons inside an atom’s nucleus are out of balance. This atom now strives to become balanced. To do this, it gives off some of its energy and particles. Or, it changes one or more of its neutrons into protons, also releasing energy. There are lots of ways the decay can happen. But the result is the same: the unstable isotope eventually becomes a new, stable one.
Morphing at a clock-like rate
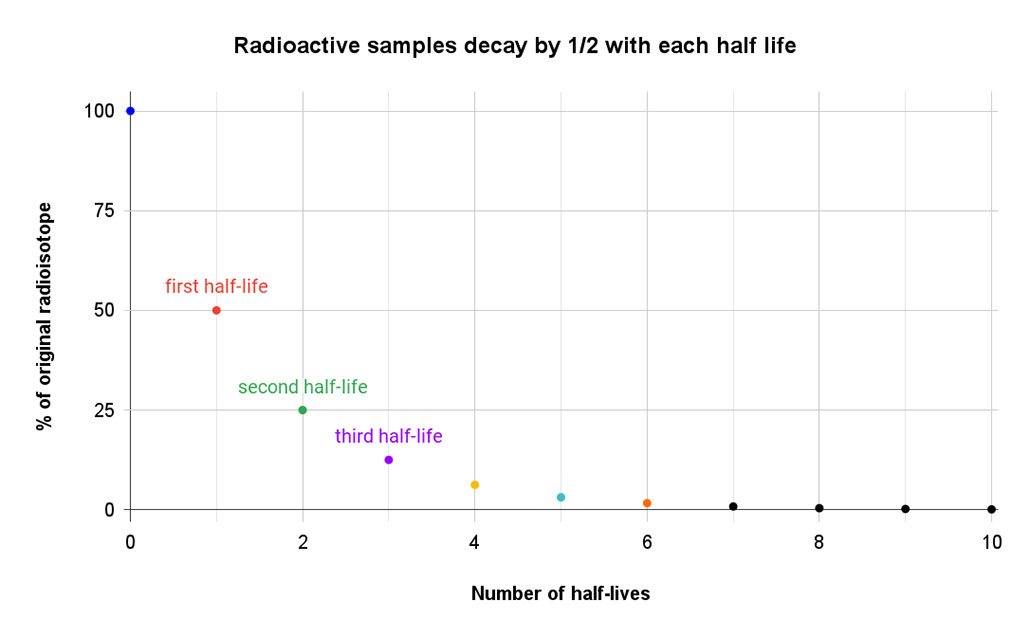
How long it takes an isotope to decay depends on a lot of factors. But scientists describe the process in terms of its half-life. An isotope’s half-life is defined as the amount of time it takes for one-half of the atoms of a radioactive isotope to decay. That half-life is always the same — like an unwritten rule — that is specific to each isotope.
If you start with 80 unstable atoms, 40 will remain at the end of the first half-life. The rest will have decayed to a new isotope. After two half-lives, just 20 atoms of the original isotope would remain. Three half-lives would leave only about 10 atoms of the original isotope. By the end of the fourth half-life, there are only five atoms of the original isotope. All of the rest have morphed into stable atoms.
Some isotopes decay very quickly. Take the lab-made isotope lawrencium-257. Its half-life is little more than a half-second. Other isotopes may have a half-life measured in hours, days or years. Then there’s the real record-holder: xenon-124. In April 2019, a team of researchers identified its half-life as 18 billion trillion years. That’s more than a trillion times the current age of our universe! (This isotope’s decay occurs as two protons in the nucleus each absorb an electron from the atom’s outer shell and then release a neutrino. This transforms both protons into neutrons and creates tellurium-128.)
Some decays involve an atom’s nucleus ejecting a single particle. Other decays may be a complicated multi-step process. For instance, sometimes one isotope ejects energy and a particle, which then results in a new unstable isotope. This interim atom now decays (with a new half-life), again shedding energy and some particles as it seeks to become stable. Still other decay chains can lead one element to morph into two or more different ones on its path to stability. For instance, uranium-238 decays into radioactive isotopes of thorium, radium, radon and bismuth — before ending up as the non-radioactive lead-206.






