Graphene’s superstrength
Scientists believe that graphene may change the world of electronics

Don’t look now, but the future of electronics may be as close as the pencil in your hand.
blackred / iStockphoto
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
Big technology comes in tiny packages. New cell phones, music players and personal computers get smaller every year, which means these electronics require even smaller components on the inside. Engineers are looking for creative ways to build these components, and they’ve turned their eyes to graphene, a superthin material that could change the future of electronics.
Graphene isn’t just small, it’s “the thinnest possible material in this world,” says Kostya Novoselov, a scientist who studies graphene at the University of Manchester, in the United Kingdom. He calls it a “wonder material.” It’s so thin that you would need to stack about 25,000 sheets just to make a pile as thick as a piece of ordinary white paper. If you were to hold a sheet of graphene in your fingers, you’d have no idea because you wouldn’t be able to see it.
In addition to being nearly invisible, graphene is also superstrong. In July, engineers at Columbia University in New York City showed that graphene is 200 times stronger than steel, making it the strongest known substance on the planet. Move over, Superman!
Graphene is made of carbon, one of the most abundant elements in the universe. Every known kind of life contains carbon; so do diamonds and coal. Graphene is a sheet of carbon, but only one atom thick. (An atom is the smallest possible piece of an element. If you change an atom of carbon, then it’s not carbon anymore.) You don’t have to look far to find graphene — it’s all around you. You can even try to find some right now.
 |
|
This is the famous Hope Diamond, housed at the Smithsonian’s National Museum of Natural History in Washington, D.C. Both diamonds and graphene are made of carbon.
|
| The Smithsonian Institution |
Do-it-yourself graphene
If you want a sneak peek of this high-tech wonderstuff, all you need is a pencil, paper and a little adhesive tape. Use the pencil to shade a small area on the paper, and then apply a small piece of adhesive tape over the area. When you pull up the tape, you’ll see that it pulls up a thin layer of some of the shading from your pencil. That layer is called graphite, one of the softest minerals in the world. When you write with a pencil, you’re actually leaving a trail of graphite on the paper.
Now stick the same piece of tape on another sheet of paper and pull the tape up — there should be an even thinner layer, this time left on the paper. Now imagine that you do this over and over, until you get the thinnest possible layer of material on the paper. This layer would be only one atom thick, and you wouldn’t be able to see it. Graphite is made of layers of graphene, so when you get to the thinnest possible layer, you’ve found graphene.
In 2004, scientists used a form of this method (in the laboratory) to isolate graphene for the first time. Those scientists included Novoselov and his Manchester colleague, Andre Geim. Their success was a huge surprise to the scientific community. Researchers had thought about graphene for a long time, but “for years, it was thought that graphene couldn’t exist,” says Jonathan Coleman, a physicist at Trinity College Dublin, in Ireland.
Since then, scientists like Coleman have been looking for new ways to make and use graphene.
 |
|
Graphite, shown here, is also made of carbon atoms. It’s one of the softest minerals in the world and is used to make tennis rackets, batteries and the “lead” in your pencil.
|
| U.S. House of Representatives |
A graphene future
Once scientists can make large amounts of graphene, it could show up in a wide range of applications. Take newspapers, for example. In the next decade or so, says Coleman, newspapers probably won’t be printed on regular paper. Instead, newspaper stories could be displayed on a kind of superthin electric paper, like a computer screen that you can carry around with you. But unlike a computer screen, this electronic paper will be durable and flexible. “You’ll be able to roll it up and fold it and put it in your back pocket,” he says. “It will be flexible and fantastic.”
Because it is strong, thin, transparent and can conduct electricity, graphene is a great candidate for this kind of device. Geim, one of the scientists who first isolated graphene, says graphene could also be used in the production of solar cells, which need materials that can both conduct electricity and let light through.
Graphene might also play a role in the future of cell phones, personal music players or even personal computers. Inside these devices are millions of transistors, tiny electrical switches that control the flow of electricity. Working together, transistors act like the “brain” of a device. The more transistors you have, the faster your computer. As computers get faster and more complicated, scientists are looking for new ways to build smaller transistors. Most transistors are made from silicon.
In early 2008, Novoselov led a team of scientists to build the world’s smallest transistor. It was made of graphene and measured only about 10 atoms across and 1 atom thick. In the laboratory, the scientists showed that the graphene transistor was faster than a silicon transistor. But there’s an interesting problem — graphene transistors are too small to be useful in everyday use! It may be years before computers can use these tiny graphene transistors, Novoselov says, but that day is coming. The future looks bright.
“The beauty of transistors made of graphene is that they can be made very small,” he says. “They will be very fast, and we are searching for ways to make them work even faster.”
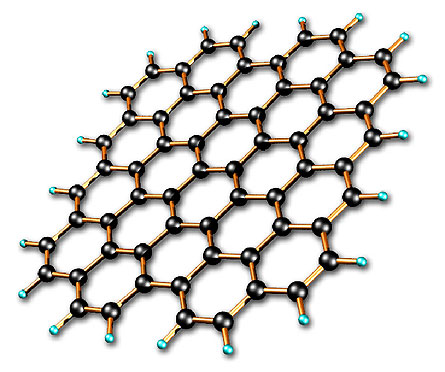 |
|
The carbon atoms that make up graphene stick together like a web made of hexagons (six-sided shapes).
|
| Lawrence Berkeley National Laboratory |
“Magic liquids” and the bumpy road ahead
Research into graphene is still in the early stages, so it could be years before we use any devices with the wonder material inside. One of the biggest problems right now is how to make large amounts of graphene. Unfortunately, you can’t buy graphene at your local hardware store. And even if you could, it comes with a hefty price tag. Superstrong and superthin, graphene is also superexpensive.
“In terms of mass, it’s the most expensive material known to man,” says Coleman. To buy an amount of graphene equal to one teeny-tiny slice of one human hair would cost you more than $1,500. “The United States as a country can afford to buy only about a milligram of graphene per year,” says Novoselov.
In August, Coleman and his team announced they had come up with a solution. They engineered an experiment where they mixed a special liquid with graphite. After they shook the mixture, they noticed that big flakes of graphene started peeling away from the graphite — and sticking to the liquid! Coleman calls such substances “magic liquids,” and so far his team has found ten different kinds. Scientists can use these liquids as an inexpensive way to produce large sheets of graphene.
There’s still a lot of work to do, he says, but “we’ve pointed the direction to where this is going.”
Going Deeper:






